Summary
Despite widespread COVID-19 vaccination, questions remain about vaccine safety and immunogenicity in vulnerable populations, such as older adults. We evaluated the safety and immunogenicity of four doses of CoronaVac in adults above and below 60 who received the first two doses in two different schedules (0–14 and 0–28 days apart). While CoronaVac demonstrated excellent safety across age groups, older adults showed reduced reactogenicity. In the 0–28 schedule, both age groups exhibited similar frequencies of SARS-CoV-2-specific CD4+ and CD8+ T cells, though memory T cell distribution patterns differed. Notably, adults over 60 showed diminished virus-neutralizing antibody responses compared to younger participants. The 0–14 schedule produced equivalent cellular and neutralizing antibody responses between age groups, albeit at lower levels than the 0–28 schedule. Our data indicate that primary vaccination schedules can influence the humoral immune responses and memory T cell distribution between age groups.
Subject areas: Immune response
Graphical abstract

Highlights
-
•
CoronaVac has an outstanding safety profile in adults
-
•
T cell responses induced by four doses of CoronaVac were comparable across age groups
-
•
In the 0–28 schedule, memory CD8+ T cells in older adults show terminal differentiation bias
-
•
Older adults showed reduced neutralizing antibodies after vaccination
Immune response
Introduction
Age is one of the most significant risk factors for severe coronavirus disease 2019 (COVID-19), with older adults being more likely to experience severe disease, hospitalization, and death following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as compared to young adults.1 This heightened vulnerability has been attributed to immunosenescence and a higher prevalence of chronic comorbidities in this population.
The rapid development and global deployment of vaccines were critical in reducing COVID–19–related morbidity and mortality.2 CoronaVac, an inactivated SARS-CoV-2 vaccine, was widely used, particularly in low and middle-income countries. It was included in the World Health Organization’s Emergency Use Listing and administered in at least 56 countries during the pandemic.3,4 In May 2023, the WHO declared the end of the pandemic phase, in part due to the widespread adoption of effective vaccination campaigns.5,6
CoronaVac has been demonstrated to be a safe and immunogenic vaccine that protects against severe COVID-19.7,8,9,10,11,12 Previous work by our group showed that a two-dose primary schedule spaced 28 days apart elicited strong neutralizing antibody responses and virus-specific CD4+ T cell activation.7,8,9,10,11,12,13,14 Booster doses further enhanced and sustained these immune responses, supporting the use of a multi-dose regimen to maintain protection over time.12,13,14
Older adults were underrepresented in many of the pivotal trials evaluating COVID-19 vaccines. Consequently, there is limited knowledge of how age influences the magnitude and quality of vaccine-induced immune responses, particularly in the context of inactivated vaccines. Several reports suggest that older adults present fewer local and systemic adverse events following vaccination but also develop lower levels of neutralizing antibodies and antigen-specific T cells compared to younger individuals.10,14,15,16 These findings raise concerns regarding vaccine efficacy in this vulnerable group.
A previous study described that older adults fully vaccinated with the mRNA-based vaccine BNT162b2 (Pfizer-BioNTech) showed lower frequencies of activation-induced marker (AIM) CD4+ Th1 cells after the first dose in those aged 60 or older compared to younger adults, and a more pronounced reduction of these cells at three months after the second vaccine dose, compared to adults younger than 60 that received standardized vaccination schedules. A similar pattern was observed regarding the humoral response, in which adults aged 60 or older showed lower IgG titers against the receptor binding domain (RBD) of SARS-CoV-2 compared to young adults.15 Supporting these findings, another study showed that the humoral response against SARS-CoV-2 induced by three different vaccines (BNT162b2 mRNA, Moderna mRNA, and Janssen Ad26.COV2.S) elicited reduced geometric mean titers of neutralizing antibodies in adults older than 65 when compared to younger adults, six months post-immunization.17 Other studies have consistently shown that adults older than 60–65 develop reduced humoral responses after vaccination compared to younger adults,18,19,20,21 evidencing a negative association between age and anti-SARS-CoV-2 antibody production.18 Subjects analyzed in these latter studies were immunized with mRNA and adenovirus-based vaccines. Even though these platforms are highly immunogenic, they are exclusively based on eliciting immune responses to the SARS-CoV-2 Spike protein as the viral antigen. In contrast, inactivated vaccines such as CoronaVac display a broader range of viral antigens. This difference in antigenic composition may shape immune responses differently across age groups, but such comparisons remain scarce.
In this study, we assessed how age and vaccination schedule influence the safety and immunogenicity of CoronaVac by comparing reactogenicity, T cell responses, and neutralizing antibodies in adults above and below 60 years old who received four vaccine doses under two primary schedules (0–14 or 0–28 days). These findings offer insights for optimizing vaccine strategies for older populations.
Results
Older adults display more comorbidities than younger participants
The number of enrolled participants was 2,302 between 19 and 78 years old (Figure 1; median 47.1, SD ± 16.5); 1,091 were women (47.4%) and 759 (33%) were health care workers. Among the 686 older adults participating (60–78 years old, median age 67.4, SD ± 6.4), 508 (74.1%) had significantly higher frequencies of chronic diseases (p < 0.001) and chronic conditions (p < 0.005), except asthma, compared to younger participants. The frequency of chronic conditions in older adults was: 44.5% arterial hypertension, 24.5% obesity, 21.4% hypothyroidism, 9.9% allergic rhinitis, 5.8% asthma, 13.3% diabetes, 4.4% cardiovascular disease, and 4.2% dyslipidemia.
Figure 1.
Volunteer enrollment, randomization, and distribution in the study
Among 2,303 enrolled volunteers, 2,302 were randomized and assigned to receive two doses of CoronaVac in a 28-day (0–28) or 14-day (0–14) interval. 1,616 volunteers were younger than 60 years old, and 686 were older than 60 years old.
The subgroup for immunogenicity analyses was recruited in one of the clinical centers (Red de Salud UC-Christus). It consisted of 239 participants immunized with four doses of CoronaVac, including 132 younger and 107 older adults for both vaccination schedules (Figure 2A). To confirm the representativeness of this subgroup, statistical comparisons were performed between participants included and non-included in this population, showing no significant differences in key variables such as age, sex, and comorbidities (p > 0.05; Tables S1 and S2). These baseline characteristics highlight significant age-related clinical differences, particularly in the prevalence of chronic diseases in older adults. This comorbidity burden will likely modulate immune responses and underscores the need to assess vaccine performance in this population subset.
Figure 2.
Schematic representation of vaccination schedules, analyses performed, and sample collection of volunteers included in the immunogenicity study
(A) Among 2,302 enrolled healthy adults, 437 were included in the immunogenicity study, and the immunogenicity results obtained for 238 and 199 volunteers from the 0–28 or 0–14 schedule, respectively, who were followed up until 12 weeks after the fourth dose, were included in the analyses. Neutralizing antibodies (nAb) were analyzed in blood samples from 233 volunteers (n = 126, 18-59 years old and n = 107, ≥60 years old) by conventional virus neutralization test (cVNT) and 187 volunteers (n = 100, 18-59 years old and n = 87, ≥60 years old) by surrogate virus neutralization test (sVNT) against ancestral SARS-CoV-2. Cellular immunity was analyzed in blood samples from 146 volunteers (n = 85, 18-59 years old and n = 61, ≥60 years old) at each time point by flow cytometry to quantify Activation Induced Markers (AIM) and 83 volunteers (n = 42, 18-59 years old and n = 41, ≥60 years old) at each time point by ELISPOT to quantify IFN-γ secreting Spot Forming Cells (SFC).
(B) Blood samples were collected before the first vaccination (pre-immune), 4 weeks after the second dose, 4 weeks after the third dose (1st booster), and 4 and 12 weeks after the fourth booster (2nd booster).
Older adults show low reactogenicity to CoronaVac
When assessing the reactogenicity to the immunization schedule, we observed that adults ≥60 years old showed a lower frequency of most of the local and systemic adverse events (AEs) after each vaccine dose compared to younger adults. Regarding the vaccination schedule, after the second dose, younger adults vaccinated under the 0–28 schedule reported fewer local AEs than those with the 0–14 schedule. On the other hand, no differences were found between the two vaccination schedules in older adults (Tables S3 and S4). Most AEs reported were mild and moderate (Grade 1 and 2) in older adults and resolved within a few days. These findings confirm the favorable tolerability profile of CoronaVac across age groups, with particularly low reactogenicity in older adults. This reduction in the frequency of AEs may reflect both immunosenescence and differences in AE perception or the reporting of these events in older populations.
CoronaVac displays a good safety profile for all age groups
Regarding the grade of the AEs upon vaccination, no Grade 4 AEs were reported in the whole study population. Similarly, no Grade 3 AEs were reported after the first two doses. After the third dose, two local (1 pain and 1 induration) and 9 systemic Grade 3 AEs were reported (5 headache and 4 fatigue); just one (fatigue) occurred in a ≥60-year-old participant. After the fourth dose, no local and 8 systemic Grade 3 AEs were reported (5 fatigue, 2 myalgias, and 1 fever); none were in older adults. On the other hand, some local and systemic AEs were reported to have a lower frequency in the 0–28 vaccination schedule than in the 0–14 schedule (Tables S3 and S4). Regarding serious adverse events (SAEs), overall, during the study, 80 SAEs were reported in 77 subjects; 40 were older adults and 37 were younger (p < 0.0001). A 63-year-old participant presented 3 SAEs and a 74-year-old presented 2 SAEs. Four deaths occurred in participants aged 21-, 51-, 60-, and 77 years, none of which were related to vaccination. These results provide reassuring evidence for the continued use of CoronaVac in all age groups.
CD4+ T cell immune response across age groups and vaccine schedules was similar
Initially, we evaluated the CD4+ T cell responses induced after each of the four doses of CoronaVac in younger and older adults enrolled in the 0–14 and 0–28 vaccination schedules. For this purpose, PBMCs were stimulated with mega-pools (MPs) of peptides derived from the ancestral SARS-CoV-2 virus, as described previously.7,8,9,13,14,22,23 The biological markers analyzed were determined by flow cytometry, and the gating strategy is shown in Figure S1.
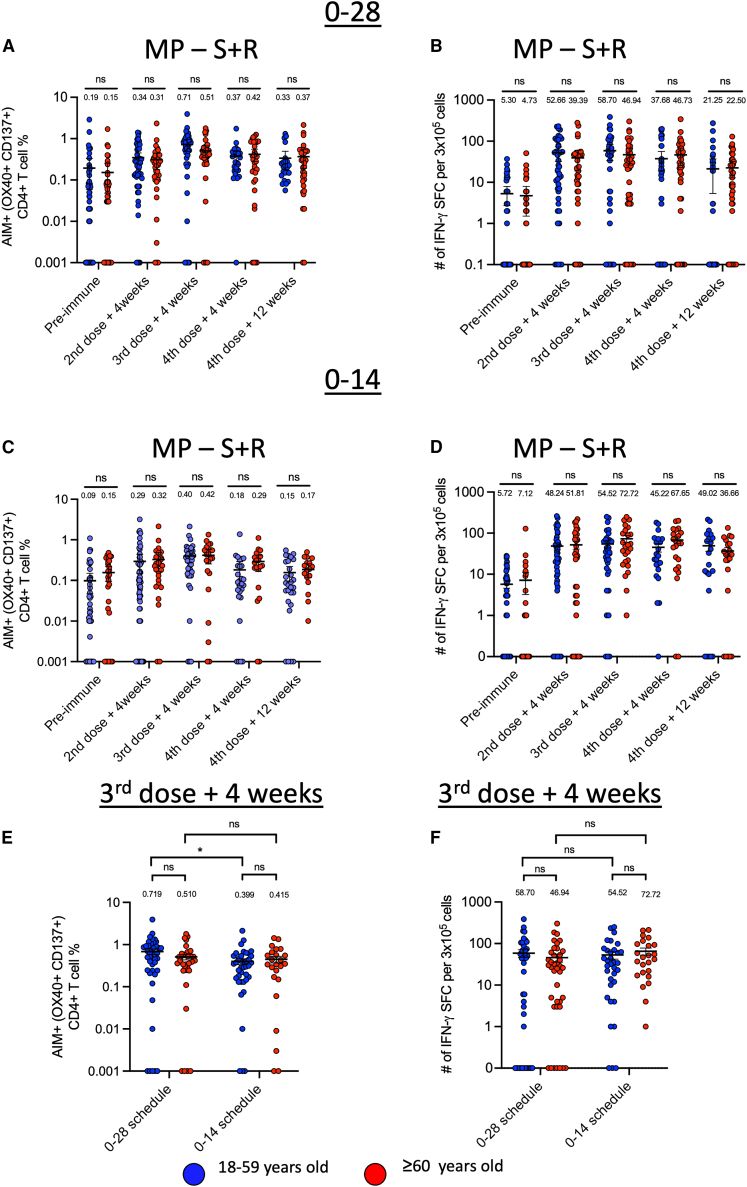
Our data show that the induction of SARS-CoV-2-specific CD4+CD137+OX40+ (AIM+CD4+) T cells (Figure 3A) and the numbers of IFN-γ-producing cells (Figure 3B) were equivalent in younger and older adults within the 0–28 schedule at each time point analyzed. Similarly, when we compared the induction of AIM+CD4+ T cells and the numbers of IFN-producing cells in younger and older adults within the schedule 0–14, we observed an equivalent frequency of activated T cells in older adults and young adults at each time point analyzed (Figure 3C). Comparable IFN-γ-producing cells were detected at each time point for both age groups within the 0–14 schedule (Figure 3D).
Figure 3.
Comparison of anti-SARS-CoV-2 CD4+ T cell response between younger and older adults immunized with CoronaVac in the 0–28 or 0–14 schedule
(A–D) Cellular responses were evaluated in isolated PBMCs from adults 18–59 years old (n = 85) and ≥60 years old (n = 61) before vaccination (pre-immune), 4 weeks after the second dose, 4 weeks after the third dose (first booster), at 4 weeks after the fourth dose (second booster), and at 12 weeks after the fourth dose (second booster). Percentages of AIM+CD4+ T cells (OX40+CD137+) and number of IFN-γ-producing spot-forming cells (SFCs) were determined by flow cytometry and ELISPOT, respectively, after stimulation with megapools of S + R peptides of PBMCs samples collected from volunteers immunized in the 0–28 (A and B) and 0–14 (C and D) schedule.
(E and F) Subsequently, a comparison of AIM+CD4+T cells (E) and the number of IFNγ-producing spot-forming cells (SFCs) (F) for both vaccination schedules was performed 4 weeks after the third dose. Horizontal lines represent means and standard error of the mean. Flow cytometry data were normalized against the DMSO control.
Data are presented as mean ± SEM. Statistical analysis was performed using a t-test, ∗ p < 0.05. Each data point represents an individual volunteer, and all measurements were obtained from a single experiment per subject.
Next, we compared the frequency of AIM+ CD4+ T cells induced by administering a third vaccine dose between vaccination schedules. Consistent with previous findings,15 young adults from the 0 to 28 schedule showed a higher percentage of AIM+CD4+ T cells in response to MP-S + R as compared with younger adults belonging to the 0–14 schedule at four weeks after the third dose (Figure 3E), however, we did not observe significant differences in the percentage of AIM+CD4+ T cells in adults older than 60 years between the two schedules (Figure 3E). Additional analyses showed no difference in IFN-γ producing cells between older adults vaccinated with 0–14 compared to 0–28 schedules after stimulation with MP–S + R (Figure 3F).
Therefore, we evaluated the frequency of AIM+ T cells specific against spike (MP-S) and the rest of the viral proteome (MP-R). Our data showed that compared to younger adults, older adults from the 0–28 schedule had equivalent frequency of AIM+CD4+ T cells specific for the spike protein of SARS-CoV-2 (Figure S2A) and the rest of the viral proteome (Figure S2B) at each analyzed time point post-immunization. Notably, when comparing the frequency of AIM+CD4+ T cells in younger and older adults belonging to the 0–14 schedule, after the fourth dose, older adults showed an elevated percentage of S-specific AIM+CD4+ T cells in comparison to younger adults (Figure S2C). However, no differences were found in the frequency of AIM+ CD4+ T cells specific for the rest of the viral proteome (Figure S2D). When we compared the response to the third dose for both schedules, an increased percentage of AIM+CD4+ T cells was observed in young adults in the 0–28 schedule when compared to the 0–14 schedule in cells that were stimulated with MP-S and MP-R (Figure S2E). Although these differences were not observed in older adults, older adults from the 0–28 schedule showed an increased tendency compared to adults from the 0–14 schedule after stimulation with MP-R alone (p = 0.06; Figure S2F). There were no significant differences in IFN-γ-producing cells between younger and older adults from the 0–28 and 0–14 schedules after stimulation with each MP (Figures S2G and S2H).
We observed that both young and older adults exhibited a peak in AIM+CD4+ T cell activation and IFN-γ production 4 weeks after the third dose in both vaccination schedules, followed by a decline at 12 weeks after the fourth dose (Figure 3). Therefore, we evaluated whether the frequency of AIM+CD4+ T cells is associated with IFN-γ production. Simple linear regression analyses revealed positive associations between AIM+CD4+T cells and IFN-γ production in young adults in the 0–28 schedule up to the 3rd dose +4 weeks (R2 = 0.41). Then, this positive association was lost (R2 = 0.01) at the 4th dose +4 weeks, compared to the previous time point. We hypothesize that this response is due to more non-responder subjects at the 4th dose +4 weeks and 12 weeks later (Figure S3A). Consistently, we observed the same trend in subjects >60 years of age, at the same time points mentioned above (Figure S3B).
As for young and older adults with the 0–14 schedule, no positive association was found between AIM+CD4+ T cells and IFN-γ production (Figures S3C and S3D). This may be due to the low frequency of IFN-γ-secreting cells observed at all time points evaluated for both age groups, but not necessarily due to a biological effect. These data indicate that CoronaVac effectively induces CD4+ T cell responses across age groups and schedules. While activation levels are comparable, the functional coupling between AIM expression and IFN-γ production appears to be diminished in older adults, suggesting age-related uncoupling of T cell activation and effector functions.
CD8+ T cell immune responses against SARS-CoV-2 were similar in younger and older adults immunized with CoronaVac
Next, we evaluated the CD8+ T cell response induced by CoronaVac in younger and older adults. PBMCs were stimulated with CD8+ T cell MPs, named MP-CD8A and MP-CD8B, to assess the expression of the activation markers CD69 and CD137 in CD8+ T cells (AIM+) and IFN-γ production by flow cytometry and ELISPOT, respectively.
Comparisons between age groups showed no significant differences in SARS-CoV-2-specific CD8+ AIM+ T cells (Figure 4A) or IFN-γ production (Figure 4B) at any evaluated time post-immunization in younger and older adults receiving either the 0–28 (Figures 4A and 4B) or 0–14 schedule (Figures 4C and 4D). Furthermore, we did not observe differences between schedules in the frequency of AIM+CD8+ T cells and the numbers of IFN-γ producing cells after administering a third vaccine dose (Figures 4E and 4F).
Figure 4.
Comparison of anti-SARS-CoV-2 CD8+ T cell response between younger and older adults immunized with CoronaVac in the 0–28 or 0–14 schedule
CD8+ responses in adults were evaluated in isolated PBMCs from adults 18–59 years old (n = 85) and ≥60 years old (n = 61) before vaccination (pre-immune), at 4 weeks after the second dose, 4 weeks after the third dose (first booster), 4 weeks after the fourth dose (second booster), and 12 weeks after the fourth dose (second booster). Percentages of AIM+ CD8+ T cells (CD69+CD137+) and number of IFN-γ-producing spot-forming cells (SFCs) were determined by flow cytometry and ELISPOT, respectively, after stimulation with CD8A + CD8B MPs of PBMCs samples collected from volunteers immunized in the 0–28 (A and B) and 0–14 (C and D) schedule, respectively.
(E and F) Subsequently, a comparison of AIM+CD8+ T cells (E) and number of IFN-γ-producing spot-forming cells (SFCs) (F) for both vaccination schedules were performed 4 weeks after the third dose. Horizontal lines represent means and standard error of the mean. Flow cytometry data was normalized against the DMSO control.
Data are presented as mean ± SEM. Statistical analysis was performed using a t-test, p < 0.05. Each data point represents an individual volunteer, and all measurements were obtained from a single experiment per subject.
Age and vaccine schedule shape memory T cell profiles
To evaluate the impact of age and primary vaccination schedule on the development of memory T cell subsets due to CoronaVac immunization, we analyzed the distribution of memory phenotypes within SARS-CoV-2-specific CD4+ and CD8+ T cells by flow cytometry. We compared them with the levels and distribution of these cells before vaccination. Following stimulation with MP-S and MP-R, CD4+ T cells in younger adults immunized with the 0–28 schedule exhibited predominantly central memory (TCM) and effector memory (TEM) phenotypes (Figures 5A and 5B). Compared to pre-immune levels, TCM cells were overall maintained over time, although TEM cells showed a consistent increase after immunizations (Figures 5A and 5B). In contrast, terminally differentiated TEMRA or naïve T cells (TN) showed a low frequency at every time point analyzed, even before vaccination. A similar behavior was observed in older adults in the 0–28 schedule (Figures 5A and 5B).
Figure 5.
Comparison of memory CD4+ T cell patterns between younger and older adults immunized with CoronaVac in the 0–28 or 0-14-day interval schedules
The memory patterns of vaccinated volunteers were evaluated in blood samples of adults 18–59 years old (n = 51) and ≥60 years old (n = 44) collected before vaccination (pre-immune), 4 weeks after the second dose, 4 weeks after the third dose (first booster), 4 weeks after the fourth dose (second booster), and 12 weeks after the second booster in PBMCs samples of volunteers that received CoronaVac in two vaccination schedules (0–14 and 0–28). Frequencies of different CD4+ T cell memory subsets were evaluated by flow cytometry, including effector memory T cells (TEM), central memory T cells (TCM), fully differentiated effector memory T cells (TEMRA), and naive cells (TN). The cells were stimulated with megapools of S or R peptides. Memory T cell frequencies were quantified based on CCR7 and CD45RA expression.
(A and B) Memory T cell frequencies for the 0–28 vaccination schedule in adults under 60 years old and over 60 years old (A) in response to S and (B) R peptides, respectively.
(C and D) The equivalent data for the 0–14 vaccination schedule in adults under 60 years old and adults over 60 years old, in response to (C) S and (D) R peptides. Flow cytometry data were normalized based on the DMSO control.
Data are presented as mean ± SEM. Statistical analysis was performed using a two-way ANOVA and post hoc Tukey, p < 0.05.
In the 0–14 schedule, younger adults also showed dominant TCM and TEM AIM+CD4+ subsets, which were sustained across booster doses (Figures 5C and 5D). The memory profile was largely comparable to that seen in subjects vaccinated with the 0–28 schedule, although we did not observe an increase in TEM frequencies upon administration of boosters. In older adults receiving the 0–14 schedule, the CD4+ T cell memory compartment displayed similar characteristics, with stable TCM and TEM populations and minimal representation of TEMRA or TN. Overall, the CD4+ T cell memory response induced by CoronaVac appeared robust and consistent across the age groups analyzed and vaccination schedules, with minor differences in memory subset distribution that may reflect age-dependent kinetics of differentiation or previous antigen exposure.
Memory CD8+ T cell subset analyses revealed more pronounced differences between age groups. In the 0–28 schedule, younger adults showed a balanced distribution of TEM and TEMRA cells, with TEM being the predominant subset at all time points post-immunization for both MP-CD8A and MP-CD8B stimulations (Figures 6A and 6B). In contrast, older adults displayed a significantly higher proportion of TEMRA cells compared to younger adults, particularly at 4 weeks after the second dose. Specifically, for MP-CD8A, the frequency of TEMRA cells in older adults was significantly increased compared to younger adults (p = 0.045; Figure 6A).
Figure 6.
Comparison of memory CD8+ T cell patterns between younger and older adults immunized with CoronaVac in the 0–28 or 0-14-day interval schedules
The memory patterns of SARS-CoV-2-specific CD8+ T cells were evaluated in blood samples of 18–59 years old (n = 51) and ≥60 years old (n = 44) collected before vaccination (pre-immune), 4 weeks after the second dose, 4 weeks after the third dose (first booster), 4 weeks after the fourth dose (second booster), and 12 weeks after the second booster in PBMCs samples of volunteers that received CoronaVac in two vaccination schedules (0–14 and 0–28). Frequencies of different CD8+ T cell memory subsets were evaluated by flow cytometry, including effector memory T cells (TEM), central memory T cells (TCM), fully differentiated effector memory T cells (TEMRA), and naive cells (TN). The cells were stimulated with megapools of S or R peptides, and the memory cell frequencies were quantified based on CCR7 and CD45RA expression.
(A and B) Memory T cell frequencies for the 0–28 vaccination schedule in adults under 60 years old and adults over 60 years old (A) in response to S and (B) R peptides, respectively.
(C and D) The equivalent data for the 0–14 vaccination schedule in adults under 60 years old and adults over 60 years old, in response to (C) S and (D) R peptides. Flow cytometry data was normalized based on the DMSO control.
Data are presented as mean ± SEM. Statistical analysis was performed using a two-way ANOVA and post hoc Tukey, p < 0.05.
In the 0–14 schedule, younger adults initially developed a predominant TEM profile, but over time, a progressive increase in TEMRA percentage was observed after 12 weeks post-fourth dose (Figures 6C and 6D). Notably, older adults consistently exhibited higher TEMRA percentages and lower TCM and TN cell proportions across all time points. For MP-CD8A stimulation, at 4 weeks after the fourth dose, TN percentages were significantly lower in older compared with younger adults (p = 0.046; Figure 6C). This depletion of the naive T cell pool, alongside the expansion of TEMRA cells, underscores the impact of aging on T cell memory formation and plasticity.
These results demonstrate that while younger adults maintain a more balanced CD8+ T cell memory profile dominated by effector memory subsets, older adults develop a skewed memory phenotype dominated by TEMRA cells, especially pronounced under the 0–28-day interval schedule. These findings suggest an age-associated shift toward terminal differentiation in CD8+ T cells, possibly driven by cumulative antigen exposure and diminished thymic output, which may impact the longevity and flexibility of cellular immunity.
Age impact over the neutralizing antibody response induced by CoronaVac
Finally, we evaluated the neutralizing capacity of anti-SARS-CoV-2 antibodies in sera of vaccinated volunteers by both a sVNT and a cVNT. Importantly, older adults from the 0–28 schedule presented reduced neutralizing antibody titers against the RBD of SARS-CoV-2 induced by the second, third, and fourth doses of CoronaVac, compared with younger adults (Figure 7A). Similar results were observed for neutralization analyses with infective virus, in which younger adults showed a better neutralizing response than those aged 60 years or older after three doses of CoronaVac in the 0–28 schedule. Similar results were observed against infectious viruses, where a lower neutralizing capacity was observed in older adults after the second and third doses compared to young adults (Figure 7B). On the other hand, sVNT data showed older adults within the 0–14 schedule presented reduced neutralizing antibody responses at 4 weeks after the second dose that was partially equivalent after the third dose as compared with younger adults with the same immunization schedule (Figure 7C). However, this response was significantly reduced in older adults at 12 weeks post-immunization with the fourth dose (Figure 7C). A reduced response at 12 weeks post-immunization with the fourth dose was also observed when neutralization analyses were performed with infectious virus (Figure 7D). Also, as described previously,7 we found that two doses of CoronaVac administered in an interval of 28 days induced higher titers of neutralizing antibodies against SARS-CoV-2 in comparison to the titers induced when the second dose was administered 14 days after the first dose (Figures 7A and 7C).
Figure 7.
Comparison of neutralizing antibody response against SARS-CoV-2 between younger and older adults immunized with CoronaVac in the 0–28 or 0–14 schedule
(A–D) The neutralizing capacity of circulating antibodies was evaluated in blood samples of adults younger and older than 60 years-old collected before vaccination (pre-immune), 4 weeks after the second dose, 4 weeks after the third dose (first booster), 4 weeks after the fourth dose (second booster), and 12 weeks after the second booster in PBMCs samples of volunteers that received CoronaVac in two vaccination schedules (0–14 and 0–28). Neutralizing capacity of serum antibodies against SARS-CoV-2 in blood samples of 85 adults younger than 60 years and 61 adults aged 60 years or older were determined by a surrogate Viral Neutralization Test (sVNT) and conventional Viral Neutralization Test (cVNT) for the 0–28 schedule (A and B) and the 0–14 schedule (C and D) expressed as IU/ml.
(E and F) Numbers on top represent the geometric mean units (GMUs), and horizontal lines represent mean and standard error of the mean. Dashed line represents the lower limit of detection (4 for sVNT). The reciprocal dilution of sera needed to prevent in vitro infection of Vero E6 cells was obtained from 126 adults younger than 60 and 107 adults aged 60 and older. Subsequently, the two schedules were compared four weeks after the third dose (E and F) and four weeks after the fourth dose (G and H), by sVNT and cVNT, respectively. Numbers on top indicate the geometric mean titers (GMTs), and horizontal lines represent means and standard error of the mean. Dashed line: limit of detection: 1 for cVNT. Red values under each significance line indicate a decrease in the means of the two compared time points. Blue values indicate an increase in the means of the compared time points. Data are presented as mean ± SEM. Statistical analysis was performed using a t-test and two-way ANOVA. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
When comparing the magnitude of the response related to neutralizing capacity at four weeks after the third and fourth dose between younger adults and older adults from each vaccination schedule, we observed that younger adults receiving the 0–28 schedule presented a higher fold-increase in the levels of neutralizing titers four weeks after the third dose when compared with the fold-increase of younger adults receiving the 0–14 schedule (Figures 7E and 7F). Remarkably, the neutralizing responses observed in older adults receiving 0–28 or 0–14 schedules showed no significant differences four weeks after the third dose (Figures 7E and 7F). We did not observe significant differences after the fourth dose in any of the age groups analyzed (Figures 7G and 7H).
Next, we performed simple linear regressions to evaluate whether the neutralizing antibody responses observed after the first two doses were associated with the responses induced with the booster doses. Younger adults from the 0–28 schedule showed a positive association between RBD neutralization at four weeks after the second dose and the antibody neutralizing response observed four weeks after the third dose (R2 = 0.20, p = 0.03; Figure S4A). However, no associations were found between the neutralizing antibody responses observed at four weeks after the second dose and 4 (R2 = 0.02, p = 0.53) or twelve weeks after the fourth dose (R2 = 0.006, p = 0.72; Figure S4A). Interestingly, in older adults receiving the 0–28 schedule, the neutralizing antibody response against SARS-CoV-2 RBD induced by two doses of CoronaVac was positively associated with the response observed at four weeks after the third dose (R2 = 0.47, p = 0.0006), 4 weeks after the fourth dose (R2 = 0.33, p = 0.006) and even at 12 weeks after the fourth dose (R2 = 0.24, p = 0.02; Figure S4B).
Even though a similar association pattern was found between the second and the third doses in younger adults from the 0–14 schedule (Figure S4C), no positive associations were found in older adults in the 0–14 schedule between the neutralizing antibody response against the RBD observed at four weeks after the second dose, with the responses analyzed at 4 weeks after the third dose, and 4 or 12 weeks after the fourth dose (Figure S4D), suggesting that when the first two doses of vaccine were administered 14 days apart, the antibody neutralizing responses induced by vaccination in each booster are not necessarily determined by the first two doses, as might occur in the 0–28 schedule.
Discussion
The COVID-19 pandemic has highlighted the critical role of vaccination in protecting the global population from life-threatening diseases. Understanding the safety and the immune response induced by vaccination is essential to designing and implementing vaccination programs to protect the population optimally during pathogen outbreaks or pandemics. This is particularly crucial for the older adult population, who are prone to decompensation of chronic conditions during infectious diseases, such as COVID-19, and their immune response induced by vaccination tends to be weaker than that of younger adults, posing challenges in terms of identifying and deploying optimal vaccines and vaccination programs for this vulnerable group.
This study comprehensively assesses age-related differences in the immunogenicity and safety of the inactivated SARS-CoV-2 vaccine CoronaVac across two vaccination schedules. By comparing cellular and humoral immune responses in adults above and below 60 years of age who received four vaccine doses, we observed important age- and schedule-dependent differences in vaccine-induced immunity. CoronaVac was well tolerated across both age groups, regardless of the vaccination schedule. Interestingly, older adults reported significantly fewer AEs, a finding consistent with prior observations in recipients of both inactivated and mRNA-based vaccines.15,18 While reduced reactogenicity may reflect immunosenescence and lower innate immune activation, it may also stem from differences in AE perception or reporting among older adult individuals. Importantly, no serious adverse events were attributed to the vaccine, reinforcing its safety in populations with chronic conditions. Although there was general concern regarding the possibility that COVID-19 vaccines could, per se, affect the fragile health of older adults,24 we did not observe any decompensation of chronic diseases in the older subjects from our cohort.
One of the strengths of our study is the inclusion of older adults. Typical underlying conditions present in this group, such as hypertension, obesity, and diabetes, may impair or downplay vaccine responses through several mechanisms, including chronic inflammation, impaired lymphoid architecture, or altered immune cell metabolism. Evidence suggests that chronic conditions, such as Diabetes Mellitus T2, insulin resistance, and arterial hypertension, may be associated with reduced vaccine-induced immunogenicity even when clinically treated and controlled.25 These chronic diseases, which are often linked to obesity, are characterized by elevated levels of systemic inflammatory biomarkers. Such inflammatory profiles have been associated with lower seroconversion rates and impaired B cell responses.26,27 Consequently, the higher prevalence of arterial hypertension and dyslipidemia observed in individuals over 60 years of age may contribute to a diminished immune response to vaccination. However, although not powered to dissect individual comorbidity effects, our findings support the need for stratified analyses in future studies.
SARS-CoV-2-specific AIM+ T cells are key to vaccine efficacy. Their ability to recognize and eliminate infected cells, as well as to modulate the immune response, makes them an important target for the development of new vaccination strategies.28 In this context, studies have shown that vaccination against COVID-19 with mRNA vaccines in adults older than 65 years of age generates a lower percentage of antigen-specific AIM+ T cells than younger adults.15 This difference may be attributed to the decreased ability of the bone marrow to generate new cells with the capacity to produce an effective immune response as we age.5 Previous studies from our laboratory report that the frequency of AIM+CD8+ T cells peak at 4 weeks after the second and fourth dose of CoronaVac in subjects without age distinction, although this study showed the existence of a decreasing trend of the frequency of these cells over time that returns close to pre-immune levels.14 Our data here is consistent with this previous report and shows similar kinetics for AIM+CD8+ T cells in both age groups, either immunized in a 0–14 or a 0–28-day schedule.
Encouragingly, our study found that the two age groups have similar responses at 4 weeks after the second dose regarding the percentage of activated CD4+ and CD8+ T cells (AIM+) or in their ability to produce IFN- in the 0–28 and 0–14 vaccination schedule. However, in adults younger than 60 years of age, the 0–28 schedule promotes higher level of Spike-specific CD4+AIM+ T cells and IFN- secreting T cells. In the case of older adults, no difference was observed among vaccination schedules. These results suggest that CD8+ T cell responses induced by CoronaVac could be largely age-independent and consistent across different vaccination schedules. Thus, while the data support the conclusion that repeated CoronaVac immunization maintains detectable CD8+ T cell responses in older adults, is that functional equivalence cannot be assumed solely based on frequency or IFN-γ levels. CD8+ T cells in older adults could be less effective due to terminal differentiation, limited expansion capacity, or reduced cytotoxic granule content. In summary, CD8+ T cell responses to CoronaVac appeared comparable between younger and older adults across vaccination schedules. However, the similarity in activation of T cells and IFN-γ production does not guarantee functional equivalence. Age-related shifts toward terminal differentiation may impair CD8+ T cell efficacy despite similar frequencies. Future studies should assess cytotoxic function and phenotypic quality to better define the protective potential of CD8+ responses in older individuals.
Another part, the generation of T cell responses, is a dynamic process. These cells undergo clonal expansion after contact with an antigen and differentiate into specialized effector and memory cells.29 Once the antigen is removed, the T cell population shrinks,30 allowing the immune system to switch from an immediate to a long-term response.31 Considering these dynamics, it is plausible that the efficacy of the interval of time between the first and second dose depends on the initial effector state of the antigen-specific T cells. However, for older adults, we did not observe differences in the percentage of AIM+ T cells and IFN-γ production among the vaccination schedules.
We also observed a positive association between the percentage of AIM+ cells and IFN-γ production after the second and third doses in adults younger than 60 years who underwent the 0–28 vaccination schedule. This association was not observed in older adults. Interestingly, no significant associations were found in either age group when the 0–14 vaccination schedule was used. These results support the notion that the interval between vaccinations may influence the immune response dynamics among different age groups. At baseline, both age groups showed a lower and similar amount of AIM+CD4+, AIM+CD8+ T cells, and IFN-γ-producing cells.
Regarding the pattern of memory cells, our data showed that adults under 60 years of age who received CoronaVac in a 0–28 schedule elicited a robust frequency of TCM CD4+ T cells after the second dose for both MP-S and MP-R. This response was not observed in the 0–14 schedule, where a lower proportion of TCM cells was observed. This finding could be associated with a lower differentiation of effector TCM into TEM before stimulation, which could be related to the shorter vaccination intervals.32 A high percentage of TEM was also observed in both schedules, which remained stable, albeit with slight variations, up to 12 weeks after the fourth dose for S-derived and R-derived antigens.
Consistent with previous findings,15 individuals older than 60 years presented a lower percentage of naive T cells in cells stimulated with Spike mega pools and the rest of the viral proteome antigens throughout the vaccination period. Interestingly, we observed a higher rate of long-lived TEM expressing CD45RA (TEMRA) specific to the remaining proteome antigens of the virus in adults aged 60 years or older, which could suggest a previous exposure to related viruses or a heterochronic immune response. Furthermore, the greater number of TEMRA cells observed in adults over 60 years of age could indicate an alteration in the composition and function of CD8+ T cells related to age, which has been previously reported.33
Finally, despite cellular response being comparable, our findings revealed significant age-related differences in antibody responses against SARS-CoV-2 depending on when the first two doses of CoronaVac were administered, 14 or 28 days apart (0–14 and 0–28 schedules). Adults aged over 60 years of age displayed a lower and delayed neutralizing antibody response when receiving the vaccine in a 0–28 schedule compared to younger adults, likely explained by a reduced pool of naive B cells and likely associated with age-related bone marrow dysfunction,19 or decreasing levels of follicular helper T cells that support the differentiation of B cells into plasmatic cells.15 These data align with observations in subjects vaccinated with mRNA vaccines in a 0–21-day interval vaccination schedule15 and with studies in older adults (55–70 years old) immunized with an inactivated yellow fever vaccine,34 which described reduced neutralizing antibody titers in older adults as compared to young adults (20–30 years old). Interestingly, we found a different effect when the first two doses of CoronaVac were administered at an interval of 14 days (0–14), where the neutralizing antibody response in younger adults was equivalent to that of older adults. These results confirm that neutralizing antibody responses to CoronaVac are attenuated in older adults, especially in the 0–28 schedule. The magnitude and durability of humoral immunity are influenced by both age and vaccination interval, underscoring the need to optimize booster strategies in older adult populations.
Previous studies have shown that intervals shorter than 3 weeks between vaccine doses can negatively impact the protective immunity against smallpox,20 while a longer interval can enhance the proliferation of specific B cells.21 A similar effect was reflected in our study, which showed lower production of antiviral-neutralizing antibodies four weeks after the second dose in younger adults, especially those undergoing the 0–14 schedule. This is possibly due to the duration of the germinal center reaction and the proliferation of memory B cells that could take several weeks or months to develop,35 which would affect their differentiation pathways at the time of secondary immunization36 and thus antibody production. Remarkably, our results show that the immunization schedule did not affect the titer of neutralizing antibodies 2 weeks after the second dose in the older adult group, supporting the notion that older adults show an impairment in B cell or follicular T cell activation after vaccination. Further studies need to be performed to confirm this hypothesis.
An additional challenge in SARS-CoV-2 vaccination is the emergence of variants. Although current vaccines have demonstrated good protection against the ancestral SARS-CoV-2 virus that emerged in Wuhan and its subsequent variants, it is essential to continuously monitor the emergence of new SARS-CoV-2 variants and evaluate their potential ability to evade the vaccine-induced immune response. The emergence of Omicron and its subvariants has underlined the need to assess current platforms and know their protection level against them. Since early 2024, new subvariants with increased infectivity and immune evasion have spread rapidly, with KP.3.1.1 and XEC37,38,39 being the most widespread in the United States. However, the effectiveness of current vaccines against these variants has not yet been determined. Our previous studies have shown that a second booster of CoronaVac results in a poor neutralizing response against the Omicron variant. Yet, it protects them from severe disease by activating AIM+CD4+ T cells and IFN-γ production to a level similar to those reached by the first booster dose.14
Overall, this study confirms the low reactogenicity, favorable safety profile, and robust immunogenicity of CoronaVac in older adults while highlighting the complexity of vaccine-induced immune responses depending on age and vaccination schedule. However, a limitation of this study is that immune monitoring was restricted to 12 weeks after the fourth vaccine dose, precluding assessment of long-term differences in antibody levels and cellular memory between age groups and vaccination schedules. Furthermore, future studies should include large, comorbidity-stratified cohorts, evaluate emerging SARS-CoV-2 variants, and include extended follow-ups to define optimal booster intervals for older adults. A deeper understanding of the interactions between age, dosing intervals, and immune response components is essential for designing effective vaccination strategies to protect vulnerable populations from infectious diseases and future pandemics.
Limitations of the study
Despite the significant findings reported in this study, several limitations must be acknowledged when interpreting the obtained results. Although the overall study cohort comprised more than 2,000 participants, immunological analyses were conducted longitudinally on a smaller subset of 239 individuals recruited from a single clinical center. This limited sample size and representativeness may constrain the generalizability of the cellular and humoral immunogenicity findings reported herein. Another noteworthy aspect is that the study did not assess the specific effects of individual comorbidities over the observed immune responses due to insufficient statistical power. Additionally, the immune assays carried out only targeted the ancestral SARS-CoV-2 strain, without evaluating immune responses to emerging variants. Finally, the relatively short follow-up period (12 weeks post–fourth dose) may limit insights into the long-term durability of the observed immune responses.
Resource availability
Lead contact
Further information and requests should be directed to and will be fulfilled by the lead contact, Dr. Alexis Kalergis (akalergis@uc.cl).
Materials availability
This study did not generate new, unique reagents.
Data and code availability
-
•
All data for all assays (ELISPOT, sVNT, cVNT, flow cytometry) are included in the text or supplemental information.
-
•
This article does not report original code.
-
•
Any additional information required for re-analyzing the data reported in this paper is available from the lead contact upon request.
Consortia
Members of the CoronaVac03CL Study Group were as follows: Andrea Ingrid Schilling Redlich, Álvaro Miguel Rojas González, María Soledad Navarrete Bello, Constanza Belén Del Rio Solis, Dinely Valeska Del Pino Lavín, Natalia Elizabeth Aguirre Concha, Franco Vega Farías, Acsa Raquel Salgado Ovalle, Thomas Quinteros, Alma Muñoz, Patricio Astudillo, Monique Nicole Le Corre, Marcela Potin Santander, Sofía Aljaro Ehrenberg, Sófia López Coloma, Tania Weil Valjalo, Gema Pérez Alarcón, Melan Peralta Kong, Consuelo Zamanillo Moreira, Paula Guzmán Merino, Francisca Aguirre Boza, Aarón Cortés Rojas, Luis Federico Bátiz, Javiera Francisca Pérez Velásquez, Karen Pamela Apablaza García, Lorena Yates Barsotti, María de los Ángeles Valdés, Bernardita Hurtado, Veronique Venteneul, Constanza Astorga, Maria Francisca Bossans, Ximena Correa, Pilar Navarro, Javiera Lagas, Paula Andrea Muñoz-Venturelli, Pablo Agustín Vial, Daniela Pavez Azurmendi, Inia Andrea Pérez Villa, Amy Lisa Riviotta, Francisca González McCowley, Francisca Pilar Urrutia Goldsack, Alejandra Isabel Del Río Weldt, Claudia Andrea del Carmen Asenjo Lobos, Bárbara Paulina Vargas Latorre, Francisca Valentina Castro Fuentes, Alejandra Patricia Acuña Rogel, Javiera Constanza Guzmán Cancino, Camila Alejandra Astudillo Griffiths, Camila Portilla Fuentes, Paulina Bustos Alarcón, Carlos Delfino Garay, Carlos M. Pérez, Pilar Espinoza, Andrea Martínez, Marcela Arancibia, Harold Romero, Cecilia Bustamante, María Loreto Pérez, Natalia Uribe, Viviana Silva, Bernardita Morice, Marco Pérez, Clara Alvarado, Marcela González, Nataly Martínez, Camila Molina, Juliette Sánchez, Daniela Fuentes Hulse, Yolanda Calvo Toro, Mariela Cepeda Corrales, Rosario Lemus Manzur, Constance Marucich Baeza, Cecilia Cornejo Beas, Paulina Donato Inostroza, Martin Lasso Barreto, María Iturrieta Meléndez, María Acuña Schlegel, Ada Cascone Scarpati, Raymundo Rojas Araya, Camila Sepúlveda Contreras, Mario Alex Contreras, Yessica Campisto Sanhueza, Pablo González Sanhueza, Zoila Quizhpi Mejias, Mariella López García, Vania Pizzeghello Salfate, and Stephannie Silva Monsalve.
Acknowledgments
This study was made possible through the generous support of various institutions: Government of Chile: The Ministry of Health, Ministry of Science, Technology, Knowledge, and Innovation, and Ministry of Foreign Affairs. Instituto de Salud Pública de Chile (ISP). PATH: Provided active support in experimental design and scientific discussion. Pontificia Universidad Católica de Chile: We thank the Vice Presidency of Research (VRI), Direction of Technology Transfer and Development (DTD), Legal Affairs Department (DAJ), School of Biological Sciences, and School of Medicine for their administrative support. Data Safety Monitoring Committee: We appreciate their oversight. Study participants: We are immensely grateful for their participation and commitment. The study was funded by: The Ministry of Health, Government of Chile, Confederation of Production and Commerce (CPC), Chile. SINOVAC Biotech. Additional support: National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIH NIAID). Millennium Institute on Immunology and Immunotherapy (ICN09_016/ICN 2021_045).
Author contributions
Conceptualization and visualization, A.M.K., S.M.B., P.A.G., Y.M.-B., H.F.P., and K.A.; methodology: H.F.P., L.R.-G., C.M., D.B.R., M.R., D.M.-T., P.P.-S., C.O., A.C., B.M.S., L.F.D., N.M.S.G., F.M.-G., J.A.S., C.I., M.U., M.S.N., A.R., R.A.F., J.F., J.M., E.R., D.W., A.G., A.S., G.Z., W.M., J.A.-F., J.V.G.-A., and A.D.; data analysis, Y.M.-B., H.F.P., L.R.-G., C.M., M.R., H.A.R., A.D., and K.A.; funding acquisition, A.M.K., S.M.B., and P.A.G.; project administration: A.M.K., S.M.B., P.A.G., and K.A.; supervised, A.M.K., S.M.B., P.A.G., H.F.P., and K.A.; writing – original draft: Y.M.-B., L.R.-G., and H.F.P.; writing – review and editing: A.M.K., S.M.B., P.A.G., Y.M.-B., L.R.-G., M.R., H.F.P., and K.A. All authors contributed to the article and approved the submitted version.
Declaration of interests
G.Z. and W.M. are SINOVAC Biotech employees that contributed to the study conceptualization (clinical protocol and eCRF design) without participating in either data analysis or interpretation. A.S. is a consultant for Gritstone Bio, Flow Pharma, ImmunoScape, Moderna, AstraZeneca, Avalia, Fortress, Repertoire, Gilead, Gerson Lehrman Group, RiverVest, MedaCorp, and Guggenheim. La Jolla Institute for Immunology (LJI) has filed for patent protection for various aspects of T cell epitope and vaccine design work. All other authors declare no conflict of interest. Authors declare that they have no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Alexa Fluor® 700 anti-human CD3 Antibody Clone OKT4 | Biolegend | Cat# 317340; RRID: AB_2563407 |
| Brilliant Violet 605™ anti-human CD4 Antibody Clone RPA-T4 | Biolegend | Cat# 300556; RRID: AB_2564391 |
| Brilliant Violet 650™ anti-human CD8a Antibody Clone RPA-T8 | Biolegend | Cat# 301042; RRID: AB_2563505 |
| Brilliant Violet 510™ anti-human CD14 Antibody Clone M5E2 | Biolegend | Cat# 301842; RRID: AB_2561379 |
| Brilliant Violet 510™ anti-human CD19 Antibody Clone HIB19 | Biolegend | Cat# 302242; RRID: AB_2561668 |
| Brilliant Violet 421™ anti-human CD45RA Antibody Clone HI100 | Biolegend | Cat# 304130; RRID: AB_10900421 |
| FITC anti-human CD197 (CCR7) Antibody Clone G043H7 | Biolegend | Cat# 353216; RRID: AB_10916386 |
| PE anti-human CD69 Antibody Clone FN50 | Biolegend | Cat# 310906; RRID: AB_314840 |
| APC anti-human CD137 (4-1BB) Antibody Clone 4-1BB | Biolegend | Cat# 309810; RRID: AB_830671 |
| PE/Cyanine7 anti-human CD134 (OX40) Antibody Clone BER-ACT35 | Biolegend | Cat# 350012; RRID: AB_10901161 |
| BD Horizon™ Fixable Viability Stain Brilliant Violet 510 | BD Biosciences | Cat# 564406; RRID: AB_2869572 |
| Chemicals, peptides, and recombinant proteins | ||
| MP-S (spike protein MPs) | La Jolla Institute | No Cat# available |
| MP-R (remaining of the viral proteome MPs without the spike protein) | La Jolla Institute | No Cat# available |
| MP-CD8A (epitope HLA class I A) | La Jolla Institute | No Cat# available |
| MP-CD8B (epitope HLA class I B) | La Jolla Institute | No Cat# available |
| Phorbol 12-Myristate 13-Acetate | Sigma-Aldrich | Cat# P8139 |
| Ionomycin calcium salt | Sigma-Aldrich | Cat# I0634 |
| Ethanol | Winkler | Cat. #2223 |
| RPMI-1640 | Cytiva | Cat #SH30255.02 |
| Critical commercial assays | ||
| Human IFN-γ/IL-4 Double-Color ELISPOT | ImmunoSpot by C.T.L | Cat#hIFNgIL4-1M-10 |
| Surrogate virus neutralization test | Genscript | Cat #L00847-A |
| Software and algorithms | ||
| GraphPad Prism 10.2.1 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Flowjo® 10.10.0 | Flowjo | https://www.flowjo.com/ |
| Other | ||
| SepMate tubes | StemCell Technologies | Cat# 86460 |
| Lymphoprep | StemCell Technologies | Cat# 07851 |
Experimental model and study participant details
Study design and subject recruitment
A total of 2,302 adult volunteers (aged 18 years or older) participated in the CoronaVac03CL trial (clinicaltrials.gov #NCT04651790) in Chile from November 2020 to January 2023. After segmenting by group age (younger than 60 years old [n=1,616] and 60 to 78 years old [n=686]) volunteers were randomized to receive the first two doses of CoronaVac® (3 μg or 600 US of inactivated SARS-CoV-2 in the presence of aluminum hydroxide as adjuvant) in a time interval of 28 (0-28 schedule) or 14 days (0-14 schedule). 1,180 volunteers were randomized to receive the 0-28 schedule, 1,083 to the 0-14 schedule, and 39 volunteers withdrew from the study. Chronic disease conditions were permitted, but subjects with decompensated diseases, as determined by medical history or physical examination, neoplastic diseases, and immunodeficiencies, were excluded from the study. A third dose of CoronaVac® was administered 20 weeks after the second dose, and a fourth dose of the same inactivated vaccine was administered 24 weeks after the third dose. Figure 1 shows the CONSORT flow diagram for this study.
The sample size (n = 2,302) was determined to ensure a probability of at least 95% detecting at least one rare adverse event (incidence ≥ 0.5%), assuming a binomial distribution. For the immunogenicity subgroup, a sample size of 238 adults (n=132 < 60 years and n=106 ≥ 60 years) was calculated based on the comparison of neutralizing antibody titers between the two age groups. We assumed a mean effect size (d = 0.5), a significance level of α = 0.05, and a power of 1 - β = 0.80. We determined that at least 64 subjects per group were required with these variables and conditions. The cohort included 132 and 107 participants, respectively, securely exceeding this requirement.
Sample population and procedures
Safety and reactogenicity: All participants were followed up to assess reactogenicity and safety according to procedures previously described10 and were included in this analysis.
Immunogenicity: A subgroup of participants provided blood samples before receiving the first dose and after each dose. The analyses included subjects who received 4 doses of CoronaVac® and had all blood samples collected (238 individuals). T cell activation was determined by analyzing the number of IFN-γ spot-forming cells (SFCs) by ELISPOT and by analyzing the expression of activation-induced markers (AIM) in CD4+ (CD137 and OX40) and CD8+ T cells (CD137 and CD69) from isolated peripheral mononuclear cells (PBMCs) following stimulation with mega-pools (MPs) of peptides derived from the ancestral SARS-CoV-2 virus. A surrogate virus neutralization test (sVNT) (Genscript Cat#L00847-A) was used to measure neutralizing antibodies against the RBD of ancestral SARS-CoV-2 in the serum of 187 subjects at each described time point. In parallel, a conventional virus neutralization test (cVNT) was completed.
Ethics statement
The Institutional Scientific and Ethical Committee of Health Sciences at Pontificia Universidad Católica de Chile reviewed and approved the study protocol (# 200708006). The Chilean Public Health Institute (Instituto de Salud Pública de Chile) also approved the execution of the trial (#24204/20). The study followed the current Tripartite Guidelines for Good Clinical Practices, the Declaration of Helsinki, and local regulations. Volunteers provided their informed consent at the time of enrollment.
Method details
Enzyme-linked immunosorbent spot (ELISPOT) assay
PBMCs were resuspended 1:10 in fresh media to remove DMSO remnants from the freezing media. Then, the cells were centrifuged for 10 min at 400×g, resuspended in fresh RPMI-1640 medium (Cytiva, cat. SH30255.02) and counted using an automated cell counter (Logos Biosystems, #L40001). Cells were adjusted to 6 × 10ˆ6 cells/mL and incubated at 37°C, 5% CO2 for 15 min. ELISPOT plates (CTL) containing PVDF membranes were activated with 15 μL of 70% ethanol (Winkler, cat. #2223), washed three times with sterile PBS, and then coated with human IFN-γ and IL-4 capture antibodies (1:250 and 1:125, respectively, CTL, ImmunoSpot). After 2 h of activation at room temperature (RT), plates were washed twice with PBS and twice with PBS-Tween 0.05%. 6 × 106 PBMCs/well in RPMI were incubated with MP-S (spike protein MPs), MP-R (remaining of the viral proteome MPs without the spike protein), MP-CD8A (epitope HLA class I A),40 MP-CD8B (epitope HLA class I B),40 DMSO (as negative control), or phorbol 12-myristate 13-acetate (PMA) (1.62 μM) (Sigma, #P8139)/ionomycin (0.6 mM) (Sigma #I0634) (as positive control) for 48 h. After the incubation time, the number of IFN-γ-producing T cells was determined by ELISPOT using ImmunoSpot technology (ImmunoSpot, #hIFNgIL4-1M-10), following the manufacturer’s instructions. Spot-forming cells (SFCs) were counted in an ImmunoSpot S6 Micro Analyzer (CTL). The values obtained from the DMSO treatment were subtracted from each stimulated sample to quantify the number of IFN-γ spot-forming cells (SFCs).
Flow cytometry
5 × 105 PBMCs/well were plated in round bottom 96-well plates and stimulated with MP-S (spike protein MPs), MP-R (remaining of the viral proteome MPs without the spike protein), MP-CD8A (epitope HLA class I A),40 MP-CD8B (epitope HLA class I B),40 DMSO (as negative control), or phorbol 12-myristate 13-acetate (PMA) (1.62 μM) (Sigma, #P8139)/ionomycin (0.6 mM) (Sigma #I0634) (as positive control) for 24 h. After stimulation PBMCs were washed and incubated for 30 min with an antibody cocktail containing the following antibodies: CD3-AF700 (clone OKT4, Biolegend 317340), CD4-BV605 (clone RPA-T4, Biolegend 300556), CD8-BV650 (clone RPA-T8, Biolegend 301042), CD14-BV510 (clone M5E2, Biolegend 301842), CD19-BV510 (clone HIB19, Biolegend 302242), CD45RA-BV421 (clone HI100, Biolegend 304130), CCR7-FITC (clone G043H7, Biolegend 353216), CD69-PE (clone FN50, Biolegend 310906), CD137-APC (clone 4-1BB, Biolegend 309810), CD134 (OX40)-PE-cy7 (clone BER-ACT35, Biolegend 350012), fixable viability dye-BV510 (BD-Horizon™, AB2869572). After incubation, samples were washed with PBS, fixed in PBS/PFA 2%, and analyzed in a BD LSR-FORTESSA X20 at the flow cytometry core at the Pontificia Universidad Católica de Chile. Following data acquisition, the samples were analyzed using FlowJo 10.10.1 (flowjo.com). The gating strategies employed for cell identification are illustrated in Figure S1. The values obtained from the DMSO treatment were deducted from each stimulated sample to quantify the frequency of AIM+CD4+ and AIM+CD8+ T cells.
Surrogate virus neutralization test (sVNT)
Neutralization of the ancestral RBD-SARS-CoV-2 by circulating antibodies was evaluated by a surrogate virus neutralization test (sVNT) (Genscript Cat #L00847-A) according to the manufacturer’s instructions. Briefly, serum samples were thawed and incubated in a round-bottom plate. Serial dilutions of 1:2 were made, beginning at a 1:4 dilution and progressing up to 1:512. Then the RBD-HRP solution was added and incubated for 30 minutes. The samples were then transferred to an ACE-2-coated plate and incubated for 15 minutes. Finally, 3 washes were performed, and the TMB solution was added and incubated for 15 minutes in the dark. Then, an H2SO4 (2N) solution was added, and the plate was read in a spectrophotometer at 450 nm. The reciprocal titers of neutralizing antibodies were expressed in international units per milliliter (IU/mL) by interpolating the absorbance data from the sVNT into a standard curve following the World Health Organization international standard 20/136 and a 4-parameter logistic model.
Conventional virus neutralization test (cVNT)
The conventional virus neutralization test (cVNT) was measured as previously described.7,8,9,13,14,22,23 Vero E6 cells (4 × 10ˆ4 cells/well) were plated in 96-well plates. 100 μl of 33782CL-SARS-CoV-2 (100 TCID50) were incubated with serial dilutions of heat-inactivated volunteer serum sample (1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, and 1:512) for 1 h at 37°C. Subsequently, the SARS-CoV-2/serum mixture was added to the plated Vero E6 cells, and the cytopathic effect was evaluated after 7 days. Serum samples from COVID-19-infected and uninfected volunteers were used as positive and negative controls, respectively.
Quantification and statistical analysis
Reactogenicity: The frequency of each solicited adverse event (AE) registered within the first 7 days after receiving each dose in both schedules are shown. Differences in the incidence of each adverse event (AE) between the two age groups were evaluated using the chi-square or Fisher’s exact test. The significance level was set at 0.05 for this and all the following assays. Safety: The frequency of Grade 3 and 4 adverse events (AEs) and serious adverse events (SAEs) related to vaccination is shown. Immunogenicity: Cellular and humoral immune responses in young adults (18-59 years) and the older adult group (≥60 years) were evaluated at each time point after vaccination and analyzed using a t-test. Cellular immune response analyses included AIM+ T cell quantification by flow cytometry and IFN−γ production by ELISPOT, while humoral immune response analyses included sVNT and cVNT. The comparison of humoral and cellular responses between the two vaccination schedules (0-14 and 0-28) at similar time points was assessed by two-way ANOVA and a post-hoc Tukey’s multiple comparison test. The comparison of memory CD4+ and CD8+ T cell subsets was completed using two-way ANOVA and a post-hoc Tukey’s multiple comparison test. The associations between neutralizing antibodies or IFN−γ production and AIM+ CD4+ T cells were analyzed using simple linear regression analysis. Parametric tests were used because the analyzed data exhibited a normal distribution (as determined by the Shapiro-Wilk test and Kolmogorov-Smirnov test). All data plotting and statistics were performed using FlowJo (version 10.7) and GraphPad Prism 10.2.1.
Additional resources
The clinical registry number is #NCT04651790. Any additional information of this clinical trial can be found through the link: https://clinicaltrials.gov/study/NCT04651790.
Published: July 21, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.113167.
Supplemental information
References
- 1.Nanda A., Vura N.V.R.K., Gravenstein S. COVID-19 in older adults. Aging Clin. Exp. Res. 2020;32:1199–1202. doi: 10.1007/s40520-020-01581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood B. The contribution of vaccination to global health: past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham B.S. Rapid COVID-19 vaccine development. Science (1979) 2020;368:945–946. doi: 10.1126/SCIENCE.ABB8923. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S., Khan S., Imran I., Al Mughairbi F., Sheikh F.S., Hussain J., Khan A., Al-Harrasi A. Vaccine development against COVID-19: study from pre-clinical phases to clinical trials and global use. Vaccines. 2021;9:836. doi: 10.3390/VACCINES9080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro H.S., Lima Neto A.S., Kahn R., Sousa G.S., Carmona H.A., Andrade J.S., Castro M.C. Impact of CoronaVac on Covid-19 outcomes of elderly adults in a large and socially unequal Brazilian city: a target trial emulation study. Vaccine. 2023;41:5742–5751. doi: 10.1016/j.vaccine.2023.07.065. [DOI] [PubMed] [Google Scholar]
- 7.Gálvez N.M., Pacheco G.A., Schultz B.M., Melo-González F., Soto J.A., Duarte L.F., González L.A., Rivera-Pérez D., Ríos M., Berrios R.V., et al. Differences in the immune response elicited by two immunization schedules with an inactivated SARS-CoV-2 vaccine in a randomized phase 3 clinical trial. eLife. 2022;11:e81477. doi: 10.7554/eLife.81477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bueno S.M., Abarca K., González P.A., Gálvez N.M.S., Soto J.A., Duarte L.F., Schultz B.M., Pacheco G.A., González L.A., Vázquez Y., et al. Safety and immunogenicity of an inactivated severe acute respiratory syndrome coronavirus 2 vaccine in a subgroup of healthy adults in Chile. Clin. Infect. Dis. 2022;75:e792–e804. doi: 10.1093/cid/ciab823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soto J.A., Melo-González F., Gutierrez-Vera C., Schultz B.M., Berríos-Rojas R.V., Rivera-Pérez D., Piña-Iturbe A., Hoppe-Elsholz G., Duarte L.F., Vázquez Y., et al. Inactivated vaccine-induced SARS-CoV-2 variant-specific immunity in children. mBio. 2022;13:e0131122. doi: 10.1128/mbio.01311-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abarca K., Iturriaga C., Urzúa M., Le Corre N., Pineda A., Fernández C., Domínguez A., González P.A., Bueno S.M., Donato P., et al. Safety and non-inferiority evaluation of two immunization schedules with an inactivated SARS-CoV-2 vaccine in adults: a randomized clinical trial. Vaccines (Basel) 2022;10:1082. doi: 10.3390/vaccines10071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balcells M.E., Le Corre N., Durán J., Ceballos M.E., Vizcaya C., Mondaca S., Dib M., Rabagliati R., Sarmiento M., Burgos P.I., et al. Reduced immune response to inactivated severe acute respiratory syndrome coronavirus 2 vaccine in a cohort of immunocompromised patients in Chile. Clin. Infect. Dis. 2022;75:e594–e602. doi: 10.1093/cid/ciac167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melo-González F., Soto J.A., González L.A., Fernández J., Duarte L.F., Schultz B.M., Gálvez N.M.S., Pacheco G.A., Ríos M., Vázquez Y., et al. Recognition of variants of concern by antibodies and T cells induced by a SARS-CoV-2 inactivated vaccine. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.747830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz B.M., Melo-González F., Duarte L.F., Gálvez N.M.S., Pacheco G.A., Soto J.A., Berríos-Rojas R.V., González L.A., Moreno-Tapia D., Rivera-Pérez D., et al. A booster dose of CoronaVac increases neutralizing antibodies and T cells that recognize delta and omicron variants of concern. mBio. 2022;13:e0142322. doi: 10.1128/mbio.01423-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Méndez C., Peñaloza H.F., Schultz B.M., Piña-Iturbe A., Ríos M., Moreno-Tapia D., Pereira-Sánchez P., Leighton D., Orellana C., Covarrubias C., et al. Humoral and cellular response induced by a second booster of an inactivated SARS-CoV-2 vaccine in adults. EBioMedicine. 2023;91 doi: 10.1016/j.ebiom.2023.104563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jo N., Hidaka Y., Kikuchi O., Fukahori M., Sawada T., Aoki M., Yamamoto M., Nagao M., Morita S., Nakajima T.E., et al. Impaired CD4+ T cell response in older adults is associated with reduced immunogenicity and reactogenicity of mRNA COVID-19 vaccination. Nat. Aging. 2023;3:82–92. doi: 10.1038/s43587-022-00343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H.S., Costa V., Racine-Brzostek S.E., Acker K.P., Yee J., Chen Z., Karbaschi M., Zuk R., Rand S., Sukhu A., et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedele G., Trentini F., Schiavoni I., Abrignani S., Antonelli G., Baldo V., Baldovin T., Bandera A., Bonura F., Clerici P., et al. Evaluation of humoral and cellular response to four vaccines against COVID-19 in different age groups: a longitudinal study. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mok C.K.P., Chen C., Zhao S., Sun Y., Yiu K., Chan T.-O., Lai H.-L., Lai K.C., Lau K.M., Ling K.C., et al. Omicron BA.1-specific T-cell responses in adults vaccinated with CoronaVac or BNT162b2 in Hong Kong: an observational cohort study. Lancet Microbe. 2023;4:e418–e430. doi: 10.1016/S2666-5247(23)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadlyana E., Setiabudi D., Kartasasmita C.B., Putri N.D., Rezeki Hadinegoro S., Mulholland K., Suryadinata H., Hartantri Y., Sukandar H., et al. BCOV21 Study Group Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia. Lancet Infect. Dis. 2023;23:545–555. doi: 10.1016/S1473-3099(22)00800-3. [DOI] [PubMed] [Google Scholar]
- 20.Frasca D., Diaz A., Romero M., Blomberg B.B. The generation of memory B cells is maintained, but the antibody response is not, in the elderly after repeated influenza immunizations. Vaccine. 2016;34:2834–2840. doi: 10.1016/j.vaccine.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson L.A., Frey S.E., El Sahly H.M., Mulligan M.J., Winokur P.L., Kotloff K.L., Campbell J.D., Atmar R.L., Graham I., Anderson E.J., et al. Safety and immunogenicity of a modified vaccinia Ankara vaccine using three immunization schedules and two modes of delivery: a randomized clinical non-inferiority trial. Vaccine. 2017;35:1675–1682. doi: 10.1016/j.vaccine.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 22.J. Snyder, and M. Root-Wiley (2021). Sinovac: COVID-19 Vaccine Tracker. https://covid19.trackvaccines.org/.
- 23.Duarte L.F., Vázquez Y., Diethelm-Varela B., Pavez V., Berríos-Rojas R., Méndez C., Riedel C.A., White J.A., Kalergis A.M., Bueno S.M., González P.A. Differential severe acute respiratory syndrome coronavirus 2–specific humoral response in inactivated virus–vaccinated, convalescent, and breakthrough-infected subjects. J. Infect. Dis. 2023;228:857–867. doi: 10.1093/infdis/jiad320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang C.-K., Lee W.-J., Peng L.-N., Meng L.-C., Hsiao F.-Y., Chen L.-K. COVID-19 vaccines in older adults. Clin. Geriatr. Med. 2022;38:605–620. doi: 10.1016/j.cger.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douxfils J., Favresse J., Bayart J.-L., Edrzej Warpechowski J., Leszczy'nska P., Leszczy'nska L., Juchnicka D., Olichwier A., Szczerbí Nski Ł., KrEtowski A.J. Assessment of the immune response in patients with insulin resistance, obesity, and diabetes to COVID-19 vaccination. Vaccines. 2023;11:1203. doi: 10.3390/VACCINES11071203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westheim A.J.F., Bitorina A.V., Theys J., Shiri-Sverdlov R. COVID-19 infection, progression, and vaccination: Focus on obesity and related metabolic disturbances. Obes. Rev. 2021;22 doi: 10.1111/OBR.13313;PAGE:STRING:ARTICLE/CHAPTER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Painter S.D., Ovsyannikova I.G., Poland G.A. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33:4422–4429. doi: 10.1016/J.VACCINE.2015.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciabattini A., Pettini E., Medaglini D. CD4+ T cell priming as biomarker to study immune response to preventive vaccines. Front. Immunol. 2013;4 doi: 10.3389/fimmu.2013.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan Y., Carrington E.M., Zhang Y., Heinzel S., Lew A.M. Life and death of activated T cells: how are they different from Naïve T cells? Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerlach C., van Heijst J.W.J., Schumacher T.N.M. The descent of memory T cells. Ann. N. Y. Acad. Sci. 2011;1217:139–153. doi: 10.1111/j.1749-6632.2010.05830.x. [DOI] [PubMed] [Google Scholar]
- 31.Brown D.M., Lee S., Garcia-Hernandez M.D.L.L., Swain S.L. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against Lethal influenza virus infection. J. Virol. 2012;86:6792–6803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolibab K., Yang A., Derrick S.C., Waldmann T.A., Perera L.P., Morris S.L. Highly persistent and effective prime/boost regimens against tuberculosis that use a multivalent modified vaccine virus ankara-based tuberculosis vaccine with interleukin-15 as a molecular adjuvant. Clin. Vaccine Immunol. 2010;17:793–801. doi: 10.1128/CVI.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J., Ahmad R., Nguyen T., Cifello J., Hemani H., Li J., Chen J., Li S., Wang J., Achour A., et al. Heterogeneity and transcriptome changes of human CD8+ T cells across nine decades of life. Nat. Commun. 2022;13:5128–5131. doi: 10.1038/s41467-022-32869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz A.R., Mälzer J.N., Domingo C., Jürchott K., Grützkau A., Babel N., Nienen M., Jelinek T., Niedrig M., Thiel A. Low thymic activity and dendritic cell numbers are associated with the immune response to primary viral infection in elderly humans. J. Immunol. 2015;195:4699–4711. doi: 10.4049/jimmunol.1500598. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F., Lanzavecchia A., Araki K., Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauthner M., Havenar-Daughton C., Sok D., Nkolola J.P., Bastidas R., Boopathy A.V., Carnathan D.G., Chandrashekar A., Cirelli K.M., Cottrell C.A., et al. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity. 2017;46:1073–1088.e6. doi: 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaku Y., Okumura K., Padilla-Blanco M., Kosugi Y., Uriu K., Hinay A.A., Chen L., Plianchaisuk A., Kobiyama K., Ishii K.J., et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024;24:e82. doi: 10.1016/S1473-3099(23)00813-7. [DOI] [PubMed] [Google Scholar]
- 38.Kaku Y., Yo M.S., Tolentino J.E., Uriu K., Okumura K., Genotype to Phenotype Japan G2P-Japan Consortium. Ito J., Sato K. Virological characteristics of the SARS-CoV-2 KP.3, LB.1, and KP.2.3 variants. Lancet Infect. Dis. 2024;24:e482–e483. doi: 10.1016/S1473-3099(24)00415-8. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q., Guo Y., Mellis I.A., Wu M., Mohri H., Gherasim C., Valdez R., Purpura L.J., Yin M.T., Gordon A., Ho D.D. Antibody evasiveness of SARS-CoV-2 subvariants KP.3.1.1 and XEC. Cell Rep. 2025;44 doi: 10.1016/J.CELREP.2025.115543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data for all assays (ELISPOT, sVNT, cVNT, flow cytometry) are included in the text or supplemental information.
-
•
This article does not report original code.
-
•
Any additional information required for re-analyzing the data reported in this paper is available from the lead contact upon request.