Abstract
Background
Type 1 diabetes mellitus (T1DM) is a disease marked by insulin deficiency and hyperglycemia, resulting from the destruction of pancreatic β-cells. The progression of T1DM is significantly influenced by oxidative stress and apoptosis. Natural compounds are highly effective in the treatment of T1DM and have multiple targets. The natural compound leonurine (SCM-198) possesses anti-inflammatory, anti-oxidant, and anti-apoptotic properties. However, the potential effect of SCM-198 in the treatment of T1DM has not been studied.
Methods
Our research aims to explore the therapeutic efficacy of SCM-198 in the context of T1DM through the integration of network pharmacology, molecular docking analyses, and both in vitro and in vivo experimental validation. In this study, we used pharmacokinetics to explore the druggability of SCM-198. The mechanisms through which SCM-198 exerts its effects on T1DM were explored using a network pharmacology approach. Subsequently, molecular docking simulations were employed to investigate the binding affinities of the core genes involved in SCM-198 treatment for T1DM. We established a mouse model of streptozotocin (STZ)-induced T1DM in vivo, and evaluated the therapeutic effect of SCM-198 on T1DM by blood glucose measurement, pathology and biochemical analysis, and further formalized the core pathway of SCM-198 regulation using STZ-damaged Min6 cells in vitro.
Results
Our findings showed that SCM-198 obeys Lipinski’s rule of five and exhibit desirable absorption, distribution, metabolism excretion and toxicity (ADMET) profiles. The core targets were significantly enriched in the apoptosis pathway. Molecular docking analysis revealed that Cysteine-aspartic Acid Protease 3 (CASP3), Tumor necrosis factor (TNF), and Matrix metallopeptidase 9 (MMP9) exhibited strong binding affinity for SCM-198. In vivo experiments, SCM-198 could not only reduce the fasting blood glucose (FBG), area under the glucose curve, blood lipid, liver function, and oxidative stress level in T1DM model mice, but also effectively improve the histopathological changes of pancreas and aorta. We further demonstrated that SCM-198 could protect Min6 cells from apoptosis by modulating the Bcl-2-associated X Protein (Bax)/B-cell Lymphoma 2 (Bcl-2)/Caspase-3 signaling cascade.
Conclusion
The present study demonstrated that SCM-198 exerts a protective effect on pancreatic β-cells in T1DM by attenuating apoptosis through inhibiting the Bax/Bcl-2/Caspase-3 signaling pathway. Therefore, SCM-198 holds great promise as a potential therapeutic candidate for T1DM.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-025-05051-1.
Keywords: Leonurine, Type 1 diabetes mellitus, Network pharmacology, Bax/Bcl-2/Caspase-3 pathway, Oxidative stress
Background
Type 1 diabetes mellitus (T1DM) is a persistent autoimmune disease caused by the immune-mediated destruction of insulin-producing β-cells in the pancreas [1]. T1DM presents with persistent hyperglycemia, impaired energy, and lipid metabolism, along with structural damage to the pancreatic islets due to progressive β-cell loss [2]. Persistent hyperglycemia, coupled with chronic an inflammatory state in T1DM patients, may lead to damage across multiple tissues and organs, resulting in various macro-vascular and microvascular complications, imposing a huge burden on society and the economy [3].
Previous studies have shown that oxidative stress and apoptosis are central to the β-cell damage observed in T1DM [4]. Oxidative stress, which refers to the imbalance between intracellular oxygen radical production and the antioxidant system, plays a crucial role in the pathogenesis of T1DM [5]. Oxidative stress plays an important role in disrupting mitochondrial function and inhibiting related signaling pathways, which ultimately leads to β-cell death [6]. Caspase-3 is a key executor of the apoptotic pathway, and it’s up-regulation in pancreatic islets from patients with T1DM highlights its role in β-cell apoptosis [7]. By identifying the role of apoptosis and oxidative stress in β-cell pathophysiology, interventions may play a positive role in attenuating the progression of T1DM or reducing its associated complications [8]. Therefore, the development of apoptosis-inhibiting antioxidant therapies or drugs to improve β-cell survival is crucial for the treatment of T1DM.
At present, there are many kinds of drugs used in clinics to control blood glucose. Metformin (Met) is the first choice of oral biguanides for diabetes patients. Met adjuvant therapy for T1DM has been proven to improve insulin sensitivity, blood glucose control, lipid levels, body composition, and vascular smooth muscle function, thereby reducing the risk of vascular complications and reversing early vascular dysfunction [9]. However, long term use of Met is related to nausea, vomiting, diarrhea, acidosis, and other side effects [10]. Consequently, there remains a need for novel therapies that not only regulate blood glucose but also preserve β-cell function, reduce metabolic disturbances, and mitigate complications associated with T1DM. With continuous research in recent years, traditional Chinese medicine has unique advantages of multiple targets and excellent therapeutic effects, which have been recognized by many patients and clinicians.
Leonurine, alternatively referred to as SCM-198, is a bioactive alkaloid derived from the plant Leonurus japonicus Houtt. It has demonstrated therapeutic efficacy in a range of medical conditions, including diabetes [11], cardiovascular and cerebrovascular diseases [12], obstetrical and gynecological disorders [13], and digestive disorders. SCM-198 has demonstrated that this compound can modulate various cellular mechanisms, such as inflammation, apoptosis, ferroptosis, pyroptosis, autophagy, and angiogenesis. These effects occur through interactions with signaling pathways, including the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), nuclear factor erythroid 2-related factor 2 (Nrf-2), mitogen-activated protein kinase (MAPK), AMP-activated protein kinase (AMPK), and Janus kinase/signal transducers and activators of transcription (JAK/STAT), among others [14–17]. Based on the pharmacological properties of SCM-198 in regulating glucose metabolism, it shows great potential in the treatment of diabetes. One study investigated the effect of SCM-198 on INS-1 cells treated with dexamethasone. The findings indicated that SCM-198 effectively reduced apoptosis, stimulated cell proliferation, and alleviated the dexamethasone-induced disruption of insulin secretion in mouse islets. Moreover, SCM-198 diminished the levels of Phospho-BCL2 associated agonist of cell death (p-Bad) and p-Akt expression [18]. A recent in vivo investigation demonstrated that the administration of SCM-198 to diabetic mice significantly reduced fasting blood glucose (FBG) levels while increasing plasma insulin levels. Moreover, SCM-198 led to a reduction in plasma triacylglycerol levels and an enhancement in high-density lipoprotein cholesterol (HDL-C) concentrations. Additionally, SCM-198 normalized the altered expression of crucial hepatic glucose metabolism enzymes, including glucokinase, phosphoenolpyruvate carboxykinase, and glucose-6-phosphatase, in an Akt-dependent signaling pathway. Furthermore, SCM-198 downregulated NF-κB and reduced the expression of inflammatory genes, including tumor necrosis factor-α (TNF-α), Interleukin (IL)−6, and IL-1β [19]. Another study indicated that SCM-198 represents a potential anti-glycation compound with the capability of preventing diabetes and its related complications through the suppression of advanced glycation end products (AGE) formation [20]. To date, no studies have been published to determine whether SCM-198 has a protective effect on damaged pancreatic β-cells in T1DM individuals.
Network pharmacology and molecular docking are considered to be the most powerful methods to explore the complex relationships between drugs, their targets and diseases [21, 22]. This study aimed to apply network pharmacology and molecular docking analysis and to investigate the protective effects of SCM-198 against pancreatic β-cell injury in streptozotocin (STZ)-induced T1DM mouse model and STZ-induced Min6 cell model to elucidate the underlying mechanisms. Because of the effectiveness of the combination therapy between anti-diabetic drugs as a strategy to achieve blood sugar control, its main advantage is to delay the progression of the disease and maintain the function of pancreatic β cells, thereby reducing the risk of diabetic complications. The combination of Met and natural products may be a superior way to improve the outcome of treating T1DM [23]. By combining SCM-198 with Met, we aim to achieve a synergistic effect: Met can address the peripheral insulin resistance, while SCM-198 targets the core pathological process of β-cell destruction, thus providing a more comprehensive treatment approach for T1DM. Therefore, in addition to exploring the alone use of SCM-198 and Met (positive control drugs), this study also evaluated the effect of Met combined with SCM-198 on T1DM mice. These findings may provide potential novel therapeutic candidates for T1DM.
Materials and methods
Drug scan and absorption, distribution, metabolism excretion and toxicity
(ADMET) profiling
Druggability testing was conducted using the Lipinski rule of five (http://www.scfbio-iitd.res.in/software/drugdesign/lipinski.jsp, accessed on April 19th, 2025). The evaluation of ADMET based properties of SCM-198 along with Met was carried out using admetSAR (http://lmmd.ecust.edu.cn/admetsar2/, accessed on April 19th, 2025) and the Swiss ADME online analyzer (http://www.swissadme.ch/, accessed on April 19th, 2025).
Network pharmacology analysis
A network pharmacology method was utilized to investigate the fundamental mechanisms by which SCM-198 could demonstrate therapeutic impacts in the treatment of T1DM. TCMSP, as a distinctive systems pharmacology platform for traditional Chinese herbal medicine, offers current, quantitative, and systemic data regarding TCM components, ADME-associated characteristics, therapeutic targets, and related diseases [24]. The potential targets of SCM-198 were identified using several databases, including TCMSP (http://www.chemsrc.com, accessed on December 27th, 2023), PubChem (https://pubchem.ncbi.nlm.nih.gov/, accessed on December 27th, 2023), PharmMapper (http://www.lilab-ecust.cn, accessed on December 27th, 2023), TargetNet (http://targetnet.scbdd.com/, accessed on December 28th, 2023), and Swiss Target Pre-diction (http://www.swisstargetprediction.ch/, accessed on December 28th, 2023). Keywords such as “type 1 diabetes mellitus”, “Diabetes Mellitus, Insulin-Dependent”, and “Diabetes Mellitus, Type 1” were employed to query the TTD (https://db.idrblab.net/ttd/, accessed on December 28th, 2023), GeneCards (https://www.genecards.org/, accessed on December 29th, 2023), and Dis OMIM (https://www.omim.org/, accessed on December 29th, 2023) databases to identify potential targets related to T1DM. All databases were restricted to the species “Homo sapiens.”
Subsequently, the common targets of SCM-198 and T1DM were identified using a Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/, accessed on December 29, 2023). A protein-protein interaction (PPI) network for these common targets was constructed utilizing the STRING database (https://string-db.org/, accessed on December 30, 2023). The results and network data were then imported into Cytoscape (version 3.9.1) for visualization. To identify hub genes involved in IGEs, the Molecular Complex Detection (MCODE) plugin was applied to determine the most relevant subclusters of strongly interacting nodes. Furthermore, the “CytoHubba” plugin was used to predict the top 51 most significant genes based on the Maximal Clique Centrality (MCC) algorithm. Finally, the genes identified in the significant MCODE module intersected with those predicted by CytoHubba, resulting in the identification of the hub genes of SCM-198 in the treatment of T1DM.
Finally, the DAVID database (https://davidbioinformatics.nih.gov/, accessed on January 6th, 2024) was utilized to conduct enrichment analyses for Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG), including an analysis of GO biological functions, which were categorized into molecular functions (MF), cellular components (CC), and biological processes (BP). The top 20 enriched GO and KEGG terms, along with significantly relevant pathways with FDR < 0.05, were displayed and chosen for further investigation.
Molecular docking
The Protein Data Bank (PDB) (www.rcsb.org, accessed on June 1th, 2025) supplied the X-ray crystal structures of the primary targets. Subsequently, MGL Tools (Version 1.5.6) was employed for the preparation of structures, encompassing tasks such as the removal of water molecules, the addition of charge and nonpolar hydrogens, and parameterization. The two-dimensional structures of SCM-198 were retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/, accessed on January 10th, 2024). It was imported into Chembio3D software (Version 14.0.0.117) for energy minimization and convert the two-dimensional structure to three-dimensional structure. Open Babel software (Version 3.1.1) was used to convert SCM-198 in sdf format to pdbqt format. The active sites of proteins were predicted using web serve DoGSiteScorer (https://proteins.plus/, accessed on June 1th, 2024) and the active pockets were placed in the box when setting up the grid box for subsequent molecular docking using AutodockTools (Version 1.5.6). Additionally, the structures were prepared using AutoDockTools. Both the prepared structures of the targets and compounds were stored in PDBQT format. Molecular docking was performed on hub targets and selected compounds using AutoDockTools. AutoDockTools, PYMOL (version 2.6.0) and web serve DoGSiteScorer (https://proteins.plus/, accessed on June 2th, 2025) were employed to analyze and visualize the conformations exhibiting strong affinities. Molecular docking was repeated at least three times, and then the binding energy and predicted binding value (pKi in µM) of SCM-198 and the core target were calculated. The results are presented as the mean ± standard deviation (SD).
Animals
A total of 42 male C57BL/6J mice, aged 4 weeks and classified as Specific-pathogen-free grade, were purchased from Beijing Weitonglihua Experimental Animal Technology Co., Ltd. (Beijing, China), under the certification number [SCXK(JING)2022-0007]. Mice were housed in a Specific-pathogen-free environment with temperature (22 ± 2 °C) and humidity (40–60% RH) with food and water readily available during a 12-hour light/dark cycle. The experimental protocols were approved by the Institutional Review Board on Animal Ethics of Binzhou Medical University (2023 − 404). This study followed the standards in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health [25].
Study design
After 7 days of adaptive feeding, the weight of each mouse was recorded to establish a baseline measurement. Subsequently, the mice were randomly divided into two groups: the control group (Con, n = 6) and the type 1 diabetes mellitus group (T1DM, n = 36). The T1DM group received an intraperitoneal injection of STZ (Meilunbio, Shanghai, China; cat.no.: #MB1227) at a dosage of 180 mg/kg. The STZ solution was configured using sodium citrate buffer (0.1 mol/L, pH = 4.5). The dosage of STZ was determined according to the study by Zhou et al. [26]. An equivalent volume of sodium citrate buffer (0.1 mol/L, pH = 4.5) was administered to the Con group. Blood samples were collected from the tail tips over a 72-hour period post-modeling, and blood glucose levels were assessed using a glucose meter (Jiuan, Tianjin, China; cat.no.:AG607). The results indicated that all mice in the T1DM group exhibited random blood glucose levels exceeding 11.1 mmol/L, confirming the successful establishment of the T1DM model [27]. Furthermore, the T1DM group mice were stratified into four different groups: type 1 diabetes mellitus (T1DM, 9 mice), leonurine (SCM-198, n = 9), metformin (Met, n = 9), and a combination group of leonurine and metformin (SCM-198 + Met, n = 9). The SCM-198 groups received intragastric administration of 200 mg/kg/day SCM-198 (Yuanyebio, Shanghai, China; cat.no.: #S27453). Considering the 4R principles of animal experimentation (Reduction, Replacement, Refinement, Responsibility), and previous studies have shown that a dose of 200 mg/kg/day SCM-198 is used because of its efficacy and safety in the treatment of disease, so we used this dose to conduct an in-depth study [20]. The Met groups received intragastric administration of 250 mg/kg/day Met (Meilunbio, Shanghai, China; cat.no.: #MC2018, n = 9). The dosage of Met was determined according to the study by Wu et al. [28]. The SCM-198 + Met group was administered both SCM-198 and Met at the aforementioned dosages. In contrast, the Con and T1DM groups were treated with an equivalent volume of physiological saline. This treatment regimen was maintained for a duration of 8 weeks.
Animal modeling
General state of mice and determination of FBG level
The general conditions of the mice were observed daily, including changes in body weight, coat glossiness, changes in diet and water intake, bedding wetness, mental status, and status before and after drug administration [29]. FBG levels were assessed in all mice before the start of the experimental modeling and after intragastric administration. Blood samples were taken from the tip of the tail following a 12-hour fasting period during which the mice were administered water. Additionally, the first drop of blood was discarded, and the suction groove of the test strip was positioned at the periphery of the blood droplet. The measurement of FBG was conducted utilizing a blood glucose meter, with the resulting values being documented accordingly.
Determination of glucose tolerance of laboratory mice
Prior to conducting the oral glucose tolerance test, FBG levels were assessed and documented. Subsequently, the body weight of each mouse was recorded, and a 40% glucose solution was administered via gavage at a dosage of 2 g/kg. Blood glucose (BG) concentrations were subsequently measured at intervals of 30, 60, 90, and 120 min using a glucose meter, with the values designated as BG0, BG30, BG60, BG90, and BG120, respectively. The area under the glucose curve (AUC) was computed employing the following equation:
 |
Collection of samples and detection of organ index
Following the administration of an adequate dose of isoflurane for anesthesia, blood samples were obtained from the eye socket of the mice and transferred into 1.5 mL centrifuge tubes. These samples were allowed to stand at room temperature for a duration of 30 min before being centrifuged at 3000 rpm for 15 min to facilitate serum separation. The resultant serum was subsequently stored at −80℃ in a refrigerator for future experimental procedures. The euthanization of the mice was performed via cervical dislocation. Subsequently, the liver and pancreatic tissues of the experimental subjects were excised, and thoroughly rinsed with normal saline, excess fluid was removed, and the tissues were weighed to determine the organ index [30].
Preparation of paraffin sections of pancreas and aorta and hematoxylin and eosin staining
Anesthetize mice using an animal anesthetic ventilator, anesthetic (isoflurane, maintenance concentration: 2%, duration: 2 min). Then, experimental mice were euthanized via cervical dislocation, after which the aortic and pancreatic tissues were excised. These tissues were subsequently washed with normal saline, allowed to drain, weighed, and then immersed in a 4% paraformaldehyde solution (Solaybao, Beijing, China; cat.no.: #P1110), for fixation. Following this, paraffin-embedded sections of the pancreas and aorta were prepared. Hematoxylin and Eosin staining was performed according to the standard protocol with the reagent hematoxylin and eosin. For pathology study of islets, each group of mice contained 3 samples, one random slice of each sample [31].
Detection of serum biochemical indicators
The frozen serum, stored in the − 80℃ freezer, was taken out and allowed to thaw on ice. After performing centrifugation at 4℃, the concentrations of serum triglycerides (TG) (Shanghai Enzymes, Shanghai, China; cat.no.: #ml092652), total cholesterol (TC) (Shanghai Enzymes, Shanghai, China; cat.no.: #ml037202), low-density lipoprotein cholesterol (LDL-C) (Shanghai Enzymes, Shanghai, China; cat.no.: #ml037825), HDL-C (Shanghai Enzymes, Shanghai, China; cat.no.: #ml064274), along with liver function biomarkers such as alanine aminotransferase (ALT) (Shanghai Enzymes, Shanghai, China; cat.no.: #ml063179) and aspartate aminotransferase (AST) (Shanghai Enzymes, Shanghai, China; cat.no.: #ml058659), were quantified based on the assay kits’ guidelines.
Determination of serum lipid peroxidation levels
Liver tissues were retrieved from a −80℃ freezer and placed on ice. A total of 100 mg from each sample was measured, sectioned into smaller fragments, and subsequently transferred into a pre-chilled 1.5 mL centrifuge tube. Following this, 900 µL of lysate was introduced, along with steel balls. The liver tissues were then homogenized utilizing a SCIENTZ-48 high-throughput tissue grinder (Ningbo, China). Centrifugation of the homogenate was performed at 12,000 rpm for 10 min at a temperature of 4 °C, and the supernatant was gently harvested for subsequent experimental procedures. The methodology for assessing serum lipid peroxidation levels adhered to the provided instructions of malondialdehyde (MDA) Assay Kits (Biyuntian, Shanghai, China; cat.no.: #S0131M).
Detection of serum total superoxide dismutase (SOD) activity
Liver tissues were retrieved from a −80℃ freezer and subsequently placed on ice. To each 10 mg of tissue, 100 µL of the SOD sample preparation solution was introduced. Following this, the tissues were minced and transferred into a pre-chilled 1.5 mL centrifuge tube. Steel balls were incorporated, and the tissues were homogenized using a SCIENTZ-48 high-throughput tissue grinder. The homogenate was then subjected to centrifugation at 12,000 rpm for 10 min at 4℃. The supernatant was carefully collected for further experimental procedures. The method employed for assessing SOD activity adhered to the provided instructions of SOD Assay Kits (Biyuntian, Shanghai, China; cat.no.: #S0101M).
Cell culture
The murine Min6 pancreatic β-cell line was generously supplied by Professor Zhu of Binzhou Medical University and maintained in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA; cat.no.: #11875093), supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA; cat.no.: #10099141) and 1% penicillin-streptomycin solution (Thermo Fisher Scientific, Waltham, MA, USA; cat.no.: #15140122). Cells were cultured in a humidified incubator at 37 °C with 5% CO2.
Cell experiment and cell viability test
To induce injury in pancreatic β-cells, Min6 cells were seeded in 96-well plates at a density of 1 × 10⁴ cells per well, incubated in RPMI-1640 medium for 24-hour. Then exposed to varying concentrations of STZ (0, 1, 2, 3, 4, 5, 6, 7, 8, and 9 mM) dissolved in sterile sodium citrate buffer (0.1 mol/L, pH = 4.5) for 24-hour. After the incubation period, cell viability was assessed using the Cell Counting Kit-8 (CCK-8; HanBio, Shanghai, China; cat.no.: #HB-CCK-8-500) assay. Briefly, 10 µL of CCK-8 reagent was added to each well containing 100 µL of RPMI-1640 medium, and the plates were incubated at 37 °C for 1-hour. The absorbance at 450 nm was measured using a Tecan Spark Microplate Reader (Männedorf, Switzerland). Based on the results, 1 mM STZ for 24-hour was selected as the optimal condition for inducing T1DM-like injury, as it significantly reduced cell viability while maintaining enough viable cells for further experiments. The same method was used to assess the effect of different concentrations of SCM-198 (5, 10, 20, 40, 60, 80, 100, and 120 µM) on cell viability. To assess the potential protective role of SCM-198 against STZ-induced cytotoxicity, Min6 cells were seeded in 96-well plates at a density of 1 × 10⁴ cells per well, incubated in RPMI-1640 medium for 24-hour. Following the establishment of the in vitro pancreatic β cell injury model, STZ-treated Min6 cells were subjected to various concentrations of SCM-198 (5, 10, 20, 40, 60, 80, and 100 µM), to determine the most effective concentration. Based on the identified optimal working concentrations of STZ (1mM) and SCM-198 (40µM), experimental groups were established, including Con, T1DM, SCM-198, Met, and SCM-198 + Met. Cells in the Con group were exposed to the RPMI-1640 medium for 32-hour. Cells in the T1DM, SCM-198, Met, and SCM-198 + Met groups were exposed to 1 mM STZ for 24-hour. Then, the cells in the T1DM group were exposed to RPMI-1640 medium for 8-hour, the cells in the SCM-198 group were exposed to 40 µM SCM-198 for 8-hour, the cells in the Met group were exposed to 5 mg/L Met for 8-hour [32], the cells in scm-198 + met group were exposed to 40 µM SCM-198 and 5 mg/L Met for 8-hour. cells were harvested for subsequent experimental procedures.
Real-time quantitative PCR analysis (RT-qPCR)
Total RNA was extracted using the RNA-easy Isolation Reagent (Vazyme, Nanjing, China; cat.no.: #R701-01), followed by reverse transcription into cDNA utilizing the HiScript III RT SuperMix for qPCR (Vazyme, Nanjing, China; cat.no.: #R323-01). The quantitative PCR was performed using SYBR qPCR Master Mix (Vazyme, Nanjing, China; cat.no.: #R323-01) on the QuantStudio 3 Flex Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). Data analysis was conducted using the ΔΔCT method, with normalization to β-actin. The primer sequences used in this study (Table 1) were designed using Primer Premier 5.0.
Table 1.
Primer sequences
| Gene name | Primer sequence |
|---|---|
| β-Actin | Forward: 5’- ATGACCCAAGCCGAGAAGG − 3’ |
| Reverse: 5’- CGGCCAAGTCTTAGAGTTGTTG − 3’ | |
| Bax | Forward: 5’- TCGTCCATCGAGGATGACTTC − 3’ |
| Reverse: 5’- AACACCACAATTAAGGCAGGG − 3 | |
| Bcl-2 | Forward: 5’- AGTGACGGCCCTTCTTACG − 3’ |
| Reverse: 5’- CCGAGGGGTAGTAGCCATC − 3 |
Western blotting
Cell samples were subjected to lysis using Western and immunoprecipitation cell lysates (Biyuntian, Shanghai, China; cat.no.: #P0013J), supplemented with a cocktail of protease and phosphatase inhibitors (Biyuntian, Shanghai, China; cat.no.: #P1046), followed by SDS-PAGE electrophoresis. Subsequently, the proteins were transferred onto polyvinylidene fluoride membranes. After blocking with 5% Bovine Serum Albumin (BSA; solarbio, Beijing, China; cat.no.: #A8850), the membranes were incubated with primary antibodies against Bcl-2-associated X Protein (Bax; 1:1000 dilution; Wanleibio, Shanghai, China; cat.no.: #WL01637), B-cell Lymphoma 2 (Bcl-2; 1:1000 dilution; Wanleibio, Shanghai, China; cat.no.: #WL01556), and Caspase-3/Cleaved caspase-3 (1:1000 dilution; Wanleibio, Shanghai, China; cat.no.: #WL102117), at 4 °C, overnight with gentle agitation. On the following day, secondary antibodies conjugated to horseradish peroxidase were applied for further incubation. The immune complexes were visualized with enhanced chemiluminescence (ECL; Labjic, Beijing, China; cat.no.: #BL520B) using the automatic chemiluminescence imaging system (Tanon 5200 Multi, Shanghai, China). β-Actin was used as the internal control. All the data were verified by three independent repeated experiments. The optical densities of the immunoblots were quantified using ImageJ software (Version 1.8.0, Wayne Rasband and collaborators, National Institutes of Health, USA) and normalized to the scanning signals of the Con group.
YO-PRO-1/PI double staining
The YP1-PRO-1/PI kit (Meilunbio, China; cat.no.: #MA0653) for apoptosis and necrosis detection was employed to assess apoptotic cell levels, following the manufacturer’s instructions. Cell density of 1.6 × 10⁵ per well was applied to 6-well plates. Cells were exposed to varying drug concentrations upon achieving 60–70% confluence. After incubation, aspirate the culture and wash the cells 1 time with PBS. Add 1 mL of YO-PRO-1/PI staining solution to each well. Incubate at 37℃ for 20 min in the dark. After the end of the incubation, the fluorescence staining effect was observed under an inverted fluorescence microscope (the positive cells stained with YO-PRO-1 were green fluorescence, Ex/Em = 491/509nm; Cells positive for PI staining are red fluorescence, Ex/Em = 535/617nm). Apoptotic cells showed green fluorescence, necrotic cells were positive for both red and green fluorescence, and living cells showed little or no fluorescence. The number of apoptotic and necrotic cells in the same field of view was counted using ImageJ software [33].
Statistical analysis
Data analysis was conducted utilizing GraphPad Prism software (Version 8.0.1, GraphPad Software, USA). Results were presented as mean ± SD. Initially, the normality of the data was assessed using the Shapiro-Wilk test, and the results confirmed that the data followed a normal distribution. Consequently, the independent samples t-test was conducted, revealing a statistically significant difference at a p-value of less than 0.05.
Results
Drug scan and ADMET profiling
The druggability of prospective pharmaceutical candidates can be assessed using Lipinski’s Rule of Five [34]. This guideline, also known as Pfizer’s Rule, establishes specific parameters, including molecular weight (Mol. WT) under 500 Daltons, a maximum of 5 hydrogen bond donors (HBDs), fewer than 10 hydrogen bond acceptors (HBAs), no more than 10 rotatable bonds (RBNs), an octanol-water partition coefficient (LogP) not exceeding 5, and a molar refractivity (A) within the range of 40 to 130. This guideline assesses the drug-likeness of a compound, suggesting its potential pharmacological efficacy and oral bioavailability in the human system, informed by its pharmacokinetic properties and physicochemical characteristics. Typically, compounds with no violations or minimal deviations (not more than one) indicate potential activity, whereas two or more violations imply restricted oral bioavailability for drug candidates [35]. Both SCM-198 and Met in our analysis adhered to Lipinski’s Rule of Five, exhibiting no violations, as presented in Table 2.
Table 2.
Drug-likeness parameters of SCM-198 and Met
| Compounds | Mol. WT (g/mol) | HBDs | HBAs | RBN | LogP | A | Violation |
|---|---|---|---|---|---|---|---|
| SCM-198 | 311.33 | 3 | 6 | 0 | 0.60 | 74 | 0 |
| Met | 126.12 | 3 | 6 | 0 | −1.38 | 37 | 0 |
Mol. WT Molecular weight, HBDs Hydrogen Bond Donors, HBAs Hydrogen Bond Acceptors, RBN Rotatable bonds, LogP octanol-water partition coefficient, A a molar refractivity
Additionally, an ADMET analysis was conducted for SCM-198 and Met, which serves as the standard drug. ADMET profiling plays a crucial role in predicting the absorption, distribution, metabolism, excretion and toxicity of potential drug molecules in medicinal chemistry, offering critical insights for drug development [36]. Drug candidates that exhibit unfavorable ADMET profiles are typically excluded from clinical trials during the drug discovery process [37]. Both SCM-198 and Met demonstrated consistent results concerning the blood-brain barrier (BBB) and human absorption. Supplementary models, including human intestinal absorption (HIA), renal organic cation transporter (ROCT), and P-glycoprotein substrate assessments, further contributed to evaluating the potential of these compounds as promising drug candidates. The ROCT substrate was predicted to have potential benefits such as reduced efflux, renal elimination, and improved bioavailability, all of which contribute to a favourable pharmacokinetic profile. Moreover, both admetSAR and Swiss ADME analyses indicated that the SCM-198 and Met were non-toxic, non-carcinogenic, non-human hepatotoxicity, non-drug induced liver injury, non-rat oral acute toxicity, non-hERG Blockers, non-skin sensitization, non-eye corrosion, non-eye irritation, and non-respiratory toxicity. The ADMET-related parameters for SCM-198 and Met (the standard antidiabetic drug) are summarized in Table 3.
Table 3.
ADMET-related parameters of SCM-198 and Met
| Compounds | SCM-198 | Met |
|---|---|---|
| Absorption | ||
| BBB | - | - |
| HIA | + | + |
| CaCo2 permeability | - | - |
| PGI | - | - |
| ROCT | - | - |
| Metabolism | ||
| CYP3A4 Substrate | - | - |
| CYP2C9 Substrate | - | - |
| CYP2D6 Substrate | - | - |
| CYP3A4 Inhibition | - | - |
| CYP2C9 Inhibition | - | - |
| CYP2C19 Inhibition | - | - |
| CYP2C19 substrate | - | - |
| CYP2D6 Inhibition | - | - |
| CYP1A2 Inhibition | - | - |
| CYP1A2 substrate | - | - |
| Toxicity | ||
| Ames toxicity | - | - |
| Carcinogens | - | - |
| Human Hepatotoxicity | - | - |
| Drug Induced Liver Injury | - | - |
| Rat Oral Acute Toxicity | - | - |
| hERG Blockers | - | - |
| Skin Sensitization | - | - |
| Eye Corrosion | - | - |
| Eye Irritation | - | - |
| Respiratory Toxicity | - | - |
BBB blood-brain barrier, HIA human intestinal absorption, PGS P-glycoprotein substrate, PGI P-glycoprotein inhibitor, ROCT renal organic cation transporter, hERG Human either-a-go-go-ralted gene, + and − represent presence and absence respectively
Investigating and evaluating the shared targets of SCM-198 in alleviating T1DM
The two-dimensional molecular structure of SCM-198 is illustrated in Fig. 1A. A total of 241 potential targets were identified for SCM-198 and 8,255 for T1DM. A total of 182 overlapping targets were recognized as potential therapeutic targets of SCM-198 in the treatment of T1DM (Fig. 1B). The PPI network for the common targets was constructed utilizing the STRING database, comprising 182 nodes and 1,543 edges. The interaction data derived from the PPI network were subsequently imported into Cytoscape for visualization (Fig. 1C). The three most significant clusters (Clusters 1, 2, and 3) were identified through sub-cluster analysis conducted with MCODE (Fig. 1D-F). Additionally, the top 57 most significant genes were identified using the MCC algorithm within the Cytoscape plug-in cytohubba (Fig. 1G). Finally, based on the previously discussed data, 51 genes were identified as hub genes contributing to the therapeutic effects of SCM-198 on T1DM (Fig. 1H-I).
Fig. 1.
Identification of overlapping targets between SCM-198 and T1DM, followed by the creation of the PPI network and identification of key hub genes. A The two-dimensional molecular structure of SCM-198. B Venn diagram depicting the overlapping targets between SCM-198 and T1DM. A total of 8,255 T1DM-related targets were mapped to 241 SCM-198 targets, leading to the identification of 182 shared targets. C The PPI network for the 182 shared targets was built using the STRING database, consisting of 182 nodes and 1,543 edges. D-F Identification of key hub genes using Cytoscape and its related plug-ins. D Cluster 1 analysis. E Cluster 2 analysis. F Cluster 3 analysis. G The network of the top 57 hub genes was identified through the CytoHubba MCC algorithm. H Venn diagram employed to identify overlapping hub genes. I The core target of SCM-198 in the treatment of T1DM
GO and KEGG pathway enrichment analyses
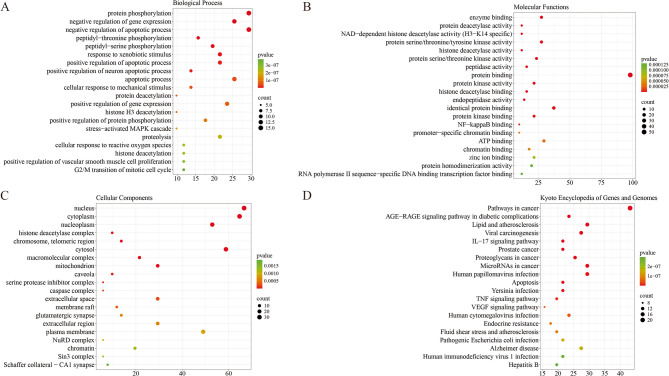
We extended the analysis to encompass 469 GO terms, including 353 BP, 49 CC, and 67 MF. The top 20 enriched GO terms suggested that a variety of cellular functions may be involved in the diverse effects of SCM-198 in treating T1DM. Within BP, prominent terms were mainly related to protein phosphorylation, inhibition of apoptotic processes, responses to xenobiotic stimuli, and cellular reactions to reactive oxygen species (ROS), among others (Fig. 2A). The candidate targets were closely associated with numerous CC, including the nucleus, cytoplasm, nucleoplasm, histone deacetylase complex, chromosomes, and telomeric regions, among others (Fig. 2B). MF were mainly characterized by enzyme binding, protein deacetylase activity, NAD-dependent histone deacetylase activity (specific to H3-K14), protein kinase activity (serine/threonine/tyrosine), and histone deacetylase activity, among others (Fig. 2C). Furthermore, KEGG pathway enrichment analysis revealed 137 significant pathways, demonstrating that SCM-198 is predominantly involved in the AGE-RAGE signaling pathway in diabetic complications, lipid metabolism and atherosclerosis, IL-17 signaling, apoptosis, Tumor necrosis factor (TNF) signaling, and vascular endothelial growth factor (VEGF) signaling pathways, among others (Fig. 2D).
Fig. 2.
A functional enrichment analysis utilizing GO and KEGG pathways was performed to identify the shared targets of SCM-198 in attenuating T1DM. A Enrichment analysis of BP related to the 182 shared targets. B Enrichment analysis of CC connected to the 182 pivotal targets. C Enrichment analysis of MF pertinent to the 182 crucial targets. D Enrichment analysis of KEGG pathways
Molecular docking
In this study, the IL-17 pathway, the apoptosis pathway, and the TNF pathway were investigated and three core proteins were screened: Cysteine-aspartic Acid Protease 3 (CASP3), TNF and Matrix metallopeptidase 9 (MMP9). CASP3, TNF, and MMP9 were molecularly docked with SCM-198 by AutodockTools-1.5.6, and the binding energy of the docking was recorded. All the results were analyzed using PyMOL Version 1.8.x software and web serve Proteins Plus for molecular docking visualization. When the binding affinity is below 0 kcal/mol, the interaction between the compound and the target protein was considered to occur spontaneously. Docking scores lower than − 3.0 kcal/mol indicate a high binding affinity between the compound and the target protein [38]. The binding energy and pKi between SCM-198 and CASP3 were − 6.29 ± 0.30 kcal/mol and 4.61 ± 0.22 µM, signifying a strong interaction. Similarly, the binding energy and pKi between SCM-198 and TNF were − 6.61 ± 0.07 kcal/mol and 4.85 ± 0.05 µM, further confirming a substantial binding affinity. Additionally, the binding energy and pKi between SCM-198 and MMP9 were − 8.63 ± 0.33 kcal/mol and 6.32 ± 0.24 µM, indicating a considerable binding affinity (Table 4). The results showed that CASP3 (Fig. 3A), TNF (Fig. 3B), and MMP9 (Fig. 3C) had high affinity and stable conformational properties for SCM-198. It is evident that CASP3, TNF, and MMP9 play a crucial role in the therapeutic effects of SCM-198 treatment in T1DM.
Table 4.
Binding energies and predicted Inhibition constant of SCM-198 with the key targets
| Target | PDB ID | Binding energies (kcal/mol) | Predicted binding value (pKi in µM) |
|---|---|---|---|
| CASP3 | 2cnn | −6.29 ± 0.30 | 4.61 ± 0.22 |
| TNF | 7kp9 | −6.61 ± 0.07 | 4.85 ± 0.05 |
| MMP9 | 4xct | −8.63 ± 0.33 | 6.32 ± 0.24 |
Fig. 3.
Molecular docking diagram and visualization of the SCM-198 and the key targets. A Visualization of docked of SCM-198-CASP3. B Visualization of docked of SCM-198-TNF. C Visualization of docked of SCM-198-MMP9
SCM-198 improves the physiological state of diabetic mice
The physical conditions of the mice across the various experimental groups are summarized in Table 5. The Con group exhibited typical eating and drinking behaviors, maintained a glossy and smooth coat, and demonstrated a steady increase in body weight. Conversely, the T1DM group displayed signs of reduced activity and cognitive decline, accompanied by a notable rise in food intake, water consumption, and urine production. Additionally, this group experienced an overall decrease in body weight, and their fur appeared disheveled, dry, and discolored. In contrast, the mice in the SCM-198, Met, and SCM-198 + Met groups exhibited moderate mental status and activity levels, with a reduction in both food and water intake compared to the T1DM group. This suggests that the administration of these two pharmacological agents may have a beneficial effect on the physical health of the mice. Notably, the fur condition of the SCM-198 + Met group was superior to that of the SCM-198 and Met groups, indicating that the combination of SCM-198 with Met has a more pronounced therapeutic effect than either agent alone.
Table 5.
Physical observation of mice in each group
| Group | Diet | Mental condition | Fur condition |
|---|---|---|---|
| Con | normal | good | bright |
| T1DM | increase | listless | Less lustrous and messy |
| SCM-198 | Different degrees of reduction | tolerableness | Under gloss |
| Met | Different degrees of reduction | tolerableness | Under gloss |
| SCM-198 + Met | Different degrees of reduction | tolerableness | tolerableness |
Effect of SCM-198 on FBG, glucose tolerance, and organ index of experimental mice
The results concerning FBG levels in mice after STZ administration are presented in Fig. 4A. The FBG levels in the T1DM, SCM-198, Met and SCM-198 + Met groups showed a significant increase compared to the Con group (p < 0.001 across all measurements), confirming successful T1DM model induction. In addition, the changes in FBG levels following 8 weeks of intragastric treatment are shown in Fig. 4B. The data demonstrate that the FBG levels in the T1DM group remained significantly higher than those in the Con group (p < 0.001), indicating ongoing hyperglycemia throughout the 8-week treatment. In contrast, FBG levels in the SCM-198, Met, and SCM-198 + Met groups exhibited a trend towards reduction compared to the T1DM group (p < 0.05, p < 0.01, and p < 0.05, respectively), indicating that all three pharmacological agents can reduce blood glucose levels. In mice, the SCM-198 and Met intervention significantly decreased glucose tolerance (Fig. 4C). Figure 4D illustrates the alterations in AUC following 8-week of intragastric administration. The AUC values for the T1DM, SCM-198, and SCM-198 + Met groups exhibited significant increases compared to the Con group (p < 0.001, for all measurements), while the Met group also demonstrated a significant increase (p < 0.01). Additionally, the AUC values for the SCM-198, Met, and SCM-198 + Met groups were significantly reduced compared to the T1DM group (p < 0.01, p < 0.01, and p < 0.05, respectively), indicating that these three treatment groups effectively mitigate the glucose tolerance in the model mice. There was a significant difference between the Met group and the SCM-198 group (p < 0.001). There was a significant difference between the Met group and the SCM-198 + Met group (p < 0.001).
Fig. 4.
Effect of SCM-198 on FBG, glucose tolerance, and organ index of experimental mice. A Changes of FBG of mice after STZ administration and B Changes of FBG in laboratory mice after gavage administration. C Oral glucose tolerance test. D AUC. E Liver index. F Pancreatic index. Note: control group (Con, n = 6), type 1 diabetes mellitus group (T1DM, n = 9), leonurine group (SCM-198, n = 9), metformin group (Met, n = 9), leonurine and metformin group (SCM-198 + Met, n = 9). Data are presented as mean ± SD of the mean. Compared with the Con group, **p < 0.01, ***p < 0.001. Compared with the T1DM group, #p < 0.05, ##p < 0.01. Compared with the SCM-198 group, &&&p < 0.001. Compared with the Met group, @@@p < 0.001
The liver and pancreatic index, a ratio that correlates organ weight with body weight, is determined by dividing the liver and pancreatin’s wet weight by the total body weight, are important indicators of an animal’ functional statuses [30]. The liver index results for each group are depicted in Fig. 4E. Significantly, the liver index was significantly higher in the T1DM, SCM-198, and SCM-198 + Met groups compared to the Con group (p < 0.001, p < 0.01, and p < 0.01, respectively). Although the Met group also exhibited an increase in the liver index compared to the Con group, this change did not reach statistical significance. Furthermore, the liver index for the SCM-198, Met, and SCM-198 + Met groups was found to be lower compared to the T1DM group, with significant differences observed solely between the Met and T1DM groups (p < 0.01). The pancreatic index results for each group are depicted in Fig. 4F. The findings indicated that the pancreatic index in the T1DM group exhibited an upward trend compared to the Con group. Additionally, compared with the T1DM group, the pancreatic index of the SCM-198 group showed a downward trend, but there was no significant difference. The pancreatic index in the Met and SCM-198 + Met groups also demonstrated an increasing trend compared to the T1DM group, but there was no significant difference.
Effects of SCM-198 on the histomorphology of pancreas and aorta in experimental mice
In addition, the morphological changes in the pancreatic tissue and aorta of the different groups of mice were examined using hematoxylin and eosin staining, as illustrated in Fig. 5. Specifically, Fig. 5A indicates that the fibrillar cells within the Con group exhibited a regular morphology, characterized by a substantial quantity of fibrillar cells systematically organized in either a round or oval configuration. The connections with adjacent acini were distinctly observable, and the nuclear architecture was well-defined, exhibiting an elliptical and uniform shape. Conversely, in the T1DM group, there was a notable reduction in the size of the islets, accompanied by a loss of their typical structure. The demarcation between the islets and surrounding acini appeared indistinct, and the arrangement of the islets was notably loose. Similarly, in the SCM-198, Met, and SCM-198 + Met groups, the delineation between the islets and peripheral acini remained unclear. Nevertheless, it is important to highlight that the pancreatic islets in the SCM-198 + Met group exhibited less damage, with larger islet cell volumes and a more orderly arrangement of islet cells compared to the SCM-198 and Met groups. This observation underscores the advantageous effects of the combination therapy involving SCM-198 and Met.
Fig. 5.
Effects of SCM-198 on histomorphology of pancreas and aorta in experimental mice. (Magnification: 10X, scale: 100 μm; 20X, scale: 50 μm)
Figure 5B illustrates a distinct delineation between the inner and outer membranes of the aorta in the Con group, where the inner membrane appears smooth. The endothelial cells exhibited a well-organized arrangement in a uniform shape, and there was no noticeable thickening of the medial membrane. Furthermore, the smooth muscle cells were arranged in a regular pattern. Conversely, in the T1DM group, the layers of the aortic wall lack clarity, and the endothelial cells exhibited signs of damage, presenting an irregular morphology. The medial membrane shows significant thickening, accompanied by a disorganized arrangement of smooth muscle cells. In the Met, SCM-198, and SCM-198 + Met groups, there was a notable improvement in endothelial cell integrity, with minimal thickening of the medial mem-brane and a regular arrangement of smooth muscle cells. These observations were particularly pronounced in the SCM-198 + Met group, further supporting the advantageous effects of the combined treatment of SCM-198 and Met (Fig. 5).
Effects of SCM-198 on the serum and hepatic biochemical parameters in experimental mice
Figure 6A illustrates the impact of SCM-198 on serum TG concentrations in experimental mice. The TG levels observed in the T1DM group were elevated in comparison to the Con group; however, significant differences were noted exclusively in the T1DM, SCM-198, and SCM-198 + Met groups compared with the Con group (p < 0.01, p < 0.01, and p < 0.05, respectively). Furthermore, a notable reduction in TG levels was recorded in both the Met and SCM-198 + Met groups relative to the T1DM group, with significant differences identified (p < 0.01 and p < 0.05, respectively). There was a significant difference between the Met group and the SCM-198 group (p < 0.05). These findings suggest that both Met and the combination of SCM-198 with Met may contribute to the regulation of TG levels.
Fig. 6.
Effects of SCM-198 on the serum and hepatic biochemical parameters in experimental mice. A TG. B TC. C HDL-C. D LDL-C. E AST. F ALT. G MDA. H SOD. I Insulin. Note: control group (Con, n = 6), type 1 diabetes mellitus group (T1DM, n = 9), leonurine group (SCM-198, n = 9), metformin group (Met, n = 9), leonurine and metformin group (SCM-198+Met, n = 9). Data are presented as mean ± standard error of the mean. Compared with the Con group, *p< 0.05, **p< 0.01, ***p< 0.001. Compared with the T1DM group, #p< 0.05, ##p< 0.01, ###p< 0.01. Compared with the SCM-198 group, &p< 0.05, &&p< 0.01, &&&p< 0.001. Compared with the Met group, @p< 0.05, @@p< 0.01, @@@p< 0.001.
The impact of SCM-198 and Met on serum TC levels in experimental mice is illustrated in Fig. 6B. Notably, TC levels exhibited a significant elevation in both the T1DM, and SCM-198 groups treatment groups compared to the Con group (p < 0.001 and p < 0.01, respectively). While the TC levels in the Met and SCM-198 + Met groups did not show a statistically significant difference from the Con group. SCM-198, Met and SCM-198 + Met groups were significantly reduced compared to the T1DM group (p < 0.05, p < 0.001, and p < 0.05, respectively). There was a significant difference between the Met group and the SCM-198 group (p < 0.01). There was a significant difference between the Met group and the SCM-198 + Met groups (p < 0.05). This suggests that both the Met and the SCM-198 are effective in normalizing TC levels.
Furthermore, the influence of SCM-198 and Met on serum HDL-C levels in experimental mice is depicted in Fig. 6C. The HDL-C concentration in the T1DM group was significantly higher than that observed in the Con group (p < 0.05). Additionally, the HDL-C levels in the Met group and the SCM-198 + Met group were significantly decreased compared to the T1DM group (p < 0.01), indicating that both Met and the combination of SCM-198 with Met can effectively modulate HDL-C levels.
Figure 6D illustrates the impact of SCM-198 and Met on serum LDL-C concentrations in experimental mice. A significant increase in LDL-C levels was observed in the T1DM group when compared to the Con group (p < 0.05). Conversely, no significant difference was observed between the treatment groups and the Con group. However, the reduction of LDL-C levels in both the SCM-198 group and the SCM-198 + Met group was significantly different from that observed in the T1DM group (p < 0.05, p < 0.01, and p < 0.05). There was a significant difference between the SCM-198 group and the Met group (p < 0.05). These findings suggest that all three treatments are effective in reducing LDL-C levels.
The impact of SCM-198 and Met on serum AST levels in experimental mice is illustrated in Fig. 6E. Notably, AST levels were significantly elevated in the T1DM, SCM-198, and SCM-198 + Met groups compared to the Con group, with statistical significance observed at p < 0.01, p < 0.05, and p < 0.01, respectively. Furthermore, a significant reduction in AST levels was observed in both the Met and SCM-198 + Met groups compared to the T1DM group (for all p < 0.05), thereby suggesting that both Met and its combination with SCM-198 are effective in lowering AST levels.
The influence of SCM-198 and Met on serum ALT levels in experimental mice is depicted in Fig. 6F. The ALT levels in the T1DM, SCM-198, and SCM-198 + Met groups were elevated compared to the Con group (p < 0.05, p < 0.01, and p < 0.01), respectively. Additionally, the Met group demonstrated a statistically significant reduction compared to the T1DM group (p < 0.05). There was a significant difference between the SCM-198 group and the Met group (p < 0.05). There was a significant difference between the Met group and the SCM-198 + Met group (p < 0.05).
The impact of SCM-198 and Met on oxidative stress levels in experimental mice is illustrated in Fig. 6G-H. Notably, MDA levels were significantly elevated in the livers of the T1DM, SCM-198, and SCM-198 + Met groups compared to the Con group (p < 0.001, p < 0.001, and p < 0.01, respectively). Conversely, the MDA levels in the SCM-198, Met, and SCM-198 + Met groups were significantly reduced in compared to the T1DM group (p < 0.01, across all measurements). This suggests that these three pharmacological agents are effective in lowering MDA levels. The MDA levels in the Met, and SCM-198 + Met groups were significantly reduced in compared to the T1DM group (p < 0.01, and p < 0.05). There was a significant difference between the Met group and the SCM-198 + Met group (p < 0.05). The influence of SCM-198 and Met on SOD levels in experimental mice is depicted in Fig. 6H. The SOD levels were significantly diminished in the T1DM, SCM-198, Met, and SCM-198 + Met groups relative to the Con group (p < 0.05, across all measurements). However, no significant alterations in SOD levels were observed in the livers of mice treated with SCM-198, Met, and SCM-198 + Met compared to the T1DM group.
The optimal concentrations of STZ and SCM-198 were determined, and the protective effects of SCM-198 on Min6 cells against STZ-induced cytotoxicity were assessed
The administration of 1 mM STZ caused substantial damage to Min6 cells, resulting in a significant reduction in cell viability compared to the Con group (P < 0.001; Fig. 7A). The treatment with SCM-198 at concentrations between 5 and 100 µM significantly enhanced Min6 cell proliferation (p < 0.01 to p < 0.001) (Fig. 7B). Considering these findings, concentrations of SCM-198 (5, 10, 20, 40, 60, 80, and 100 µM) and STZ (1 mM) were selected for subsequent testing of SCM-198’s protective effects against STZ-induced damage to Min6 cells. In Min6 cells treated with STZ, administration of SCM-198 significantly improved cell viability (p < 0.05), particularly at a concentration of 40 µM (Fig. 7C).
Fig. 7.
SCM-198 demonstrates protective effects on Min6 cells against cell death induced by STZ. A Results of the CCK-8 assay following a 24-hour treatment with STZ alone. B Results of the CCK-8 assay after a 24-hour treatment with SCM-198 alone. C Results of the CCK-8 assay after a 24-hour STZ treatment followed by an 8-hour exposure to SCM-198. Note: control group (Con, n = 6), type 1 diabetes mellitus group (T1DM, n = 6). Data are presented as mean ± standard error of the mean. Compared with the Con group, *p < 0.05, **p < 0.01, ***p < 0.001. Compared with the T1DM group, #p < 0.05
SCM-198 effectively suppressed the apoptotic process in Min6 cells induced by STZ
To further explore the anti-apoptotic effects of SCM-198 on STZ-induced pancreatic β-cells (Min6) in vitro, YO-PRO-1/PI double staining was employed. The results from the YO-PRO-1/PI double staining demonstrated that the number of cells exhibiting red fluorescence from PI labeling and green fluorescence from YO-PRO-1 labeling progressively increased in Min6 cells treated with STZ. Compared to the T1DM group, apoptosis levels were significantly reduced after treatment with SCM-198, Met, or the combination of SCM-198 and Met. The level of apoptosis was significantly reduced in the SCM-198 + Met group after treatment compared with the SCM-198 and Met groups (p < 0.05 and p < 0.01, respectively). (Fig. 8).
Fig. 8.
Effect of SCM-198 on apoptosis in Min6 cells. A Apoptosis figure after YO-PRO-1/PI staining (Magnification: 10X, scale: 100 μm). B Apoptosis statistics. C Necrotic statistics. Note: control group (Con, n = 3), type 1 diabetes mellitus group (T1DM, n = 3), leonurine group (SCM-198, n = 3), metformin group (Met, n = 3), leonurine and metformin group (SCM-198 + Met, n = 3). Data are presented as mean ± standard error of the mean. Comparison with the Con group, *p < 0.05, ***p < 0.001. Comparison with the T1DM group, ###p < 0.001. Compared with the Met group, @@p <0.01. Compared with the SCM-198 group, &p < 0.05
SCM-198 suppresses the Bax/Bcl-2/Caspase-3 signaling cascade, thereby preventing apoptosis in Min6 cells
RT-qPCR and Western blot analyses were performed to explore the essential components of the classical Bax/Bcl-2/Caspase-3 signaling cascade to gain a deeper understanding of the molecular mechanisms underlying the anti-apoptotic effects of SCM-198 in vitro. RT-qPCR analysis revealed that the expression of the pro-apoptotic gene Bax was significantly upregulated in the T1DM group compared to the Con group (p < 0.01), whereas the anti-apoptotic gene Bcl-2 was significantly downregulated in the T1DM group (p < 0.01) (Fig. 9A-C). Additionally, Western blot analysis was employed to evaluate the expression of the pro-apoptotic proteins Bax and cleaved Caspase-3, along with the anti-apoptotic protein Bcl-2 across all groups (Fig. 9D-K). The protein levels of Bax and cleaved Caspase-3 were significantly increased in the T1DM group compared to the Con group (p < 0.001, across all measurements), while Bcl-2 protein expression was significantly decreased (p < 0.05). SCM-198 treatment effectively counteracted these changes in the STZ-induced Min6 cells.
Fig. 9.
SCM-198 attenuates apoptosis in STZ-induced pancreatic β-cells (Min6) under in vitro conditions. A-B RT-qPCR was conducted to measure the mRNA ex-pression levels of Bax and Bcl-2 in all experimental conditions. C The Bcl-2 to Bax mRNA expression ratio was calculated. D Western blot images illustrating the protein expression levels of Bax and Bcl-2 in the respective experimental conditions. E-F The corresponding bar graphs present the quantification of protein expression levels. G The ratio of Bcl-2 to Bax protein expression was determined. H Western blot images showing the expression of Caspase-3 and cleaved Caspase-3 in the respective experimental groups. I-J The bar graphs display the quantification of protein expression levels. K The ratio of Caspase-3 to cleaved Caspase-3 protein levels was evaluated. Note: control group (Con, n = 3), type 1 diabetes mellitus group (T1DM, n = 3), leonurine group (SCM-198, n = 3), metformin group (Met, n = 3), leonurine and metformin group (SCM-198 + Met, n = 3). Data are presented as mean ± standard error of the mean. Comparison with the Con group, *p < 0.05, **p < 0.01, ***p < 0.001. Comparison with the T1DM group, #p < 0.05, ##p < 0.01. Compared with the SCM-198 group, &p < 0.05, &&p < 0.01. Compared with the Met group, @p < 0.05
Discussion
The pathogenesis of T1DM involves autoimmune destruction of pancreatic β-cells leading to absolute deficiency of insulin secretion [39]. Although subcutaneous insulin injection is the routine clinical treatment for T1DM, it has failed to improve patients’ quality of life [40]. Therefore, there is an urgent need to explore more effective therapies for T1DM. Numerous studies have shown that oxidative stress and apoptosis play a role in causing pancreatic islet injury in T1DM. SCM-198 exhibited a wide range of pharmacological properties, including antioxidant, anti-inflammatory, and anti-apoptotic activities. SCM-198 exhibits antioxidative properties through the enhancement of the activity of crucial antioxidant enzymes, such as SOD and catalase (CAT), as well as reducing the concentration of lactic acid dehydrogenase (LDH) [41]. Treatment with SCM-198 led to enhanced cell viability and an increased Bcl-2/Bax ratio, accompanied by a reduction in LDH activity, creatine kinase levels, and the incidence of apoptosis, as well as the ratio of cleaved-caspase-3 to pro-caspase-3 in primary cardiac myocytes under hypoxic conditions [42]. Furthermore, some studies have showed that SCM-198 demonstrated protective effects on neurons against toxicity induced by 6-hydroxydopamine in both in vitro and in vivo models through the inhibition of oxidative stress and apoptosis [43]. Therefore, it is hypothesized that SCM-198 may have a protective effect against T1DM. This investigation assessed the protective effects of SCM-198 on metabolic dysfunctions and pancreatic injury in T1DM mice. Our results demonstrate that SCM-198 not only enhances glycemic regulation and lipid metabolism but also exerts therapeutic effects in T1DM by modulating the Bcl-2/Bax/Caspase-3 signaling axis, consequently protecting pancreatic β-cells.
An in-silico analysis was performed to assess the druggability and ADMET properties of SCM-198. Drug candidates exhibiting poor ADMET profiles are typically excluded from further clinical trial consideration in drug discovery [44]. SCM-198 adhered to Lipinski’s Rule of Five, demonstrating drug-likeness while exhibiting favorable ADMET properties and no observable toxic or carcinogenic risks. Network pharmacology offers an innovative method for investigating traditional Chinese medicinal herbs [45] SCM-198 had the characteristics of multiple targets using network pharmacology analysis. Network pharmacology analysis identified the apoptosis signaling cascade as a pivotal mechanism driving SCM-198’s effects. Molecular docking revealed strong binding affinities of SCM-198 for targets such as CASP3, indicating direct interactions that regulating this pathway.
Pancreatic β-cells play a significant role in the progression of T1DM, thus making therapies aimed at mitigating β cell damage an optimal approach for managing T1DM and its associated complications. STZ, a compound known for its spectral antibiotic properties and its highly reactive methyl-nitrourea group, serves as a traditional agent in T1DM animal models, where it exerts cytotoxic effects by interacting with glucose molecules [46]. Therefore, in the present study, we developed an STZ-induced mouse model for in vivo experiments and an STZ-induced Min6 cell model for in vitro analysis, to investigate the potential effects of SCM-198 on T1DM.
Our research results revealed that there was no significant difference in insulin secretion levels between the T1DM group and the control group, which was different from the previous research results [47]. It may be related to the damage of STZ to β-cell stress to release insulin, while the reduction in insulin levels by Met may be due to its improvement in insulin sensitivity and reduction in β-cell load [48, 49]. The in vivo results indicated that the administration of SCM-198 could effectively improve the symptoms of T1DM, such as polydipsia, polyphagia, polyuria and weight loss [50]. Lowering blood glucose to normal levels is the basis for treating T1DM [51]. Insulin resistance in diabetes is usually positively related to the level of blood lipids, and dyslipidemia will promote insulin secretion and damage islet β-cells producing [9, 52]. The results of our study showed that HDL-C levels were increased in the T1DM group compared to the control group, which is different from the results of previous studies [53]. It may be related to the dysregulation of lipid metabolic pathways in T1DM mice [54]. Although the concentration of HDL-C increases, the anti-inflammatory and antioxidant ability of HDL may be impaired, and its normal protective effect on T1DM is lost. Our findings demonstrated that the intervention of SCM-198 and Met could effectively reduce TG, LDL-C in T1DM mice. In addition, the disorder of lipid metabolism increases the risk of diabetic macroangiopathy [55]. As a chronic disease, T1DM affects multiple organs, including the pancreas, liver, and blood vessels. The intervention of SCM-198 attenuated the symptoms of hyperglycemia and dyslipidemia in T1DM mice and ameliorated the lesions of the pancreas and large blood vessels. This shows that SCM-198 combined with Met has a more significant regulatory effect on blood lipid metabolism, which has a significant potential impact on the prevention and treatment of T1DM and diabetic macroangiopathy.
AST and ALT were released into the bloodstream when liver cells and mitochondria were damaged, leading to a significant increase in serum AST and ALT levels, indicating severe liver function damage [56]. The results of this study showed that SCM-198 could significantly reduce the serum ALT and AST levels in T1DM model mice, suggesting that SCM-198 has protective effects on the liver, improves liver injury in T1DM model mice, and alleviates the disorder of lipid metabolism.
Continuously elevated blood glucose levels cause oxidative stress and a series of inflammatory cascade reactions in the body, leading to an imbalance in the homeostasis of the body’s oxidative and antioxidant systems, and excessive production of ROS, which exceeds the normal scavenging capacity of the body, resulting in oxidative damage to tissues and organs, especially damage to mitochondria [57]. Oxidative stress can damage pancreatic β-cells, causing a decrease in the activity of antioxidant enzymes in the cells, resulting in a decrease in the antioxidant capacity of pancreatic β-cells and an increase in the sensitivity to ROS, which not only cause damage to the DNA and proteins in the cells, leading to apoptosis of pancreatic β-cells, but also play an important role in regulating the process of insulin secretion, which indirectly inhibits the function of β-cells [58]. Therefore, oxidative stress is widely recognized as a major factor in the pathogenesis of diabetes. Our study showed elevated MDA levels in T1DM mice. This biomarker is associated with oxidative stress, which represents the product of free radical-induced lipid peroxidation. In contrast, SCM-198 intervention suppressed MDA levels and subsequently oxidative stress in T1DM mice.
By the time T1DM is diagnosed, approximately 70–80% of the β-cell population is either impaired or destroyed by β-cell apoptosis [59]. Mitochondria are the main site of ROS production in cells, and intracellular ROS can be used as signaling molecules to regulate physiological functions in vivo [60]. Hyperglycemia-induced ROS accumulation can lead to a decrease in mitochondrial membrane potential and an increase in membrane permeability [61]. The apoptotic factor cytochrome c is released into the cytoplasm to initiate the activation of the caspase cascade (Caspase-9 and Caspase-3 are activated sequentially), leading to chromosome aggregation and DNA fragmentation [8]. Caspase-3 proteins are essential for cellular function, and play a key role in the complex programmed cell death process known as apoptosis [62]. Bcl-2 family proteins, including anti-apoptotic proteins such as Bcl-2 and Bcl-xL, and pro-apoptotic proteins such as Bax and Bad, regulate this process [63]. Dysregulation of Bcl-2/Bax, characterized by aberrant expression of either anti-apoptotic or pro-apoptotic genes, initiates apoptosis and ultimately leads to organ damage. The development of T1DM is attributed to autoimmune destruction of pancreatic islet β-cells, where islet activation stimulates antigen-presenting cells, triggering activation of CD4 helper T-cells and subsequent release of chemokines/cytokines [64]. Activation of CD8 cytotoxic T cells is subsequently induced by these cytokines, leading to subsequent destruction of β-cells. Apoptotic pathways in T1DM include both exogenous (receptor-mediated) and endogenous (mitochondria-driven) mechanisms. Exogenous pathways include the CD4-CD8-interacting Fas pathway, whereas endogenous pathways are characterized by a delicate balance between anti-apoptotic Bcl-2 and Bcl-xL proteins and pro-apoptotic Bax and Bad proteins [65]. Therefore, apoptosis-targeted interventions may effectively improve the prognosis of T1DM. SCM-198 inhibited IL-1β-induced Bax levels, increased Bcl-2 levels, and inhibited caspase-3 activity in chondrocytes, indicating its anti-apoptotic effect [66]. Our in vitro experimental results showed that SCM-198 significantly reduced apoptosis in Min6 cells. Treatment with STZ resulted in a reduction of Bcl-2 expression while increasing Bax levels, thus disrupting the Bax/Bcl-2 ratio, which significantly enhanced Caspase-3 activation [67]. In our research, SCM-198 was able to significantly reduce the expression of Bax and cleaved Caspase-3 while increasing the levels of Bcl-2 in STZ-induced cellular models. These findings indicate that SCM-198 may induce islet protection through modulation of the Bax/Bcl-2/Caspase-3 signaling pathway. And SCM-198 combined with Met showed better potential in reducing apoptosis of Min6 cells.
However, our study has some limitations. First, no in vivo molecular biology experiments were performed to evaluate the target of action and signaling pathway of SCM-198 for the treatment of T1DM. In the future, the expression of key proteins in the Bax/bcl2/caspase3 signaling pathway should be verified in vivo and pharmacological inhibition of this signaling pathway should be performed to determine whether blocking these mechanisms affects the therapeutic effect of SCM-198. Second, in in vivo experiments, SCM-198 or Met alone was more effective in reducing FBG levels, AUC, and lipid metabolism than the combination of SCM-198 and Met. As our study only assessed the effect during 8 weeks of treatment, SCM-198 in combination with Met may show a more significant benefit in the long term. And the doses of SCM-198 and Met used in our study were determined based on previous studies. However, the current dosage combination may not be optimal for maximizing the lipid-lowering effect, and there may be specific ratios or dosage ranges of these two drugs that result in greater synergistic effects on glucose and lipid metabolism. Future experiments should be designed with different drug concentration gradients of SCM-198 to explore the optimal dosing concentration and duration of dosing. Finally, there is a lack of in-depth studies on how SCM-198 binding to Caspase-3 affects its activation, future studies should employ advanced structural biology techniques, such as cryo-electron microscopy or X-ray crystallography, to accurately identify SCM-198’s binding site on Caspase-3 and perform site-directed mutagenesis experiments to disrupt Caspase-3’s proposed binding site to evaluate the effect of SCM-198 on the inhibition of Caspase-3 activation.
Conclusions
Using network pharmacology, our research identifies the potential mechanisms through which SCM-198 may target T1DM. SCM-198 exerted therapeutic effects by targeting key genes, particularly CASP3. SCM-198 can improve the blood glucose and lipid metabolism, liver oxidative stress, and pathological damage of the pancreas and aorta in T1DM mice. In vitro models of T1DM clearly show that SCM-198 plays a crucial role in protecting pancreatic β-cells against damage caused by STZ through the inhibition of the Bax/Bcl-2/Caspase-3 signaling pathway. These results not only provide compelling evidence for the role of SCM-198 in the treatment of T1DM and its complications, but also for one of its potential mechanisms of action.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- AMPK
AMP-activated protein kinase
- AUC
Area under the glucose curve
- Bax
Bcl-2-associated X Protein
- Bcl-2
B-cell Lymphoma 2
- BP
Biological processes
- CASP3
Cysteine-aspartic acid protease 3
- CC
Cellular components
- FBG
Fasting blood glucose
- GO
Gene ontology
- HDL-C
High density lipoprotein cholesterol
- IL
Interleukin
- JAK/STAT
Janus kinase/signal transducers and activators of transcription
- KEGG
Kyoto encyclopedia of genes and genomes
- LDH
Lactic acid dehydrogenase
- LDL-C
Low-density lipoprotein cholesterol
- MAPK
Mitogen-activated protein kinase
- MCC
Maximal clique centrality
- MCODE
Molecular complex detection
- MDA
Malondialdehyde
- Met
Metformin
- MF
Molecular functions
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- MMP9
Matrix metallopeptidase 9
- Nrf-2
Nuclear factor erythroid 2–related factor 2
- PI3K/Akt/mTOR
Phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin
- PDB
Protein data bank
- PPI
Protein-protein interaction
- p-Bad
Phospho-BCL2 associated agonist of cell death
- ROS
Reactive oxygen species
- RT-qPCR
Real-time quantitative PCR
- SCM-198
Leonurine
- SOD
Superoxide dismutase
- STZ
Streptozotocin
- TC
Total cholesterol
- TG
Triglycerides
- TNF
Tumor necrosis factor
- T1DM
Type 1 diabetes mellitus
- VEGF
Vascular endothelial growth factor
Authors’ contributions
ZL, FW Data curation. ZL, XL Methodology. ZL, XL Software. ZL, XL Writing – original draft. WJ, ZX Supervision. WJ, ZX Writing – review & editing. ZX Project administration. ZL and XL contributed equally to this work thus should be considered as co-first authors.
Funding
This work was supported by Major Basic Research Project of [Natural Science Foundation of Shandong Province] under Grant [ZR2021QH305], and [Startup Foundation for Introducing Talent of Binzhou Medical University] under Grant [BY2020KYQD24].
Data availability
The datasets used and analyzed during the current study are publicly available from the following databases and repositories: the Lipinski rule of five (http://www.scfbio-iitd.res.in/software/drugdesign/lipinski.jsp), the admetSAR (http://lmmd.ecust.edu.cn/admetsar2/), the Swiss ADME online analyzer (http://www.swissadme.ch/), the TCMSP (http://www.chemsrc.com), the PubChem (https://pubchem.ncbi.nlm.nih.gov/), the PharmMapper (http://www.lilab-ecust.cn), the TargetNet (http://targetnet.scbdd.com/), the Swiss Target Pre-diction (http://www.swisstargetprediction.ch/), the TTD (https://db.idrblab.net/ttd/), the GeneCards (https://www.genecards.org/), the Dis OMIM (https://www.omim.org/), the TCMSP database (https://old. tcmsp-e.com/tcmsp.php), the STRING database (https://string-db.org/), the DAVID database (https://davidbioinformatics.nih.gov/), the Protein Data Bank (www.rcsb.org), the PubChem (https://pubchem.ncbi.nlm.nih.gov/), and the DoGSiteScorer (https://proteins.plus/). The data for this study are included within the manuscript. Additional data are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The animal study protocol was conducted following the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of Binzhou Medical University (approval number: 2023 − 404, date:2023.01.12).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhiqi Li and XiaoRan Liu contributed equally to this work.
References
- 1.Foster TP, Bruggeman BS, Haller MJ. Emerging immunotherapies for disease modification of type 1 diabetes. Drugs. 2025;85(4):457–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diane A, Allouch A, Mu-U-Min RBA, Al-Siddiqi HH. Endoplasmic reticulum stress in pancreatic β-cell dysfunctionality and diabetes mellitus: a promising target for generation of functional hPSC-derived β-cells in vitro. Front Endocrinol (Lausanne). 2024;15:1386471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syed FZ. Type 1 diabetes mellitus. Ann Intern Med. 2022;175(3):ITC33–48. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Garijo A. When dying is not the end: apoptotic caspases as drivers of proliferation. Semin Cell Dev Biol. 2018;82:86–95. [DOI] [PubMed] [Google Scholar]
- 5.Prasad MK, Mohandas S, Ramkumar KM. Dysfunctions, molecular mechanisms, and therapeutic strategies of pancreatic β-cells in diabetes. Apoptosis. 2023;28(7–8):958–76. [DOI] [PubMed] [Google Scholar]
- 6.Darenskaya MA, Kolesnikova LI, Kolesnikov SI. Oxidative stress: pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med. 2021;171(2):179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi D, Schroer SA, Lu SY, Cai EP, Hao Z, Woo M. Redundant role of the cytochrome c-mediated intrinsic apoptotic pathway in pancreatic β-cells. J Endocrinol. 2011;210(3):285–92. [DOI] [PubMed] [Google Scholar]
- 8.Yavuz O, Dincel GC, Yildirim S, El-Ashram S, Al-Olayan E. Impact of apoptosis and oxidative stress on pancreatic beta cell pathophysiology in streptozotocin-induced type 1 diabetes mellitus. Tissue Cell. 2024;91:102552. [DOI] [PubMed] [Google Scholar]
- 9.Khadilkar A, Oza C, Mondkar SA. Insulin resistance in adolescents and youth with type 1 diabetes: a review of problems and solutions. Clin Med Insights Endocrinol Diabetes. 2023;16:11795514231206730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrivastava S, Sharma A, Saxena N, Bhamra R, Kumar S. Addressing the preventive and therapeutic perspective of Berberine against diabetes. Heliyon. 2023;9(11):e21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manickasamy MK, Daimary UD, Sajeev A, Abbas M, Alqahtani MS, Abdulhammed A, Kunnumakkara AB. Comprehensive review of leonurine: Harnessing its therapeutic potential for chronic diseases. Naunyn Schmiedebergs Arch Pharmacol. 2025. [DOI] [PubMed]
- 12.Meng Y, Xi T, Fan J, Yang Q, Ouyang J, Yang J. The Inhibition of FTO attenuates the antifibrotic effect of leonurine in rat cardiac fibroblasts. Biochem Biophys Res Commun. 2024;693:149375. [DOI] [PubMed] [Google Scholar]
- 13.Lin YK, Li YY, Li Y, Li DJ, Wang XL, Wang L, Yu M, Zhu YZ, Cheng JJ, Du MR. SCM-198 prevents endometriosis by reversing low autophagy of endometrial stromal cell via balancing ERα and PR signals. Front Endocrinol (Lausanne). 2022;13:858176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi L, Chen X, Pan Y, Zha Z, Tang M, Shi C, Yang B, Wang H. Leonurine exerts a protective effect in dextran sodium sulfate-induced experimental inflammatory bowel disease mice model. Gen Physiol Biophys. 2022;41(1):43–51. [DOI] [PubMed] [Google Scholar]
- 15.Liao L, Gong L, Zhou M, Xue X, Li Y, Peng C. Leonurine ameliorates oxidative stress and insufficient angiogenesis by regulating the PI3K/Akt-eNOS signaling pathway in H2O2-Induced HUVECs. Oxid Med Cell Longev. 2021;2021:9919466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao Y, Luo Y, Sun Y, Jiang J, Li Z, Wang Z, Wang M, Gu X. Leonurine exerts Anti-Inflammatory effects in lipopolysaccharide (LPS)-Induced endometritis by modulating mouse JAK-STAT/PI3K-Akt/PPAR signaling pathways. Genes (Basel). 2024;15(7):857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Chen K, Rose P, Zhu YZ. Natural products in drug discovery and development: synthesis and medicinal perspective of leonurine. Front Chem. 2022;10:1036329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu W, Cui J, Zhou F, Bai M, Deng R, Wang W. Leonurine protects against dexamethasone-induced cytotoxicity in pancreatic β-cells via PI3K/Akt signaling pathway. Biochem Biophys Res Commun. 2020;529(3):652–8. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Xin H, Liu X, Xu Y, Wen D, Zhang Y, Zhu YZ. Novel anti-diabetic effect of SCM-198 via inhibiting the hepatic NF-κB pathway in db/db mice. Biosci Rep. 2012;32(2):185–95. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Yang X, Peng A, Wang H, Lei X, Zheng L, Huang K. Inhibitory effect of leonurine on the formation of advanced glycation end products. Food Funct. 2015;6(2):584–9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Zhang D, Zhou W, Wang L, Wang B, Zhang T, Li S. Network pharmacology: towards the artificial intelligence-based precision traditional Chinese medicine. Brief Bioinform. 2023;25(1): bbad518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigby SP. Uses of molecular Docking simulations in elucidating synergistic, additive, and/or Multi-Target (SAM) effects of herbal medicines. Molecules. 2024;29(22):5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CW, You BH, Yim S, Han SY, Chae HS, Bae M, Kim SY, Yu JE, Jung J, Nhoek P, Kim H, Choi HS, Chin YW, Kim HW, Choi YH. Change of Metformin concentrations in the liver as a pharmacological target site of Metformin after long-term combined treatment with ginseng berry extract. Front Pharmacol. 2023;14:1148155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems Pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council (US). Committee for the update of the guide for the care and use of laboratory animals. guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 26.Zhou B, Li Q, Wang J, Chen P, Jiang S. Ellagic acid attenuates Streptozocin induced diabetic nephropathy via the regulation of oxidative stress and inflammatory signaling. Food Chem Toxicol. 2019;123:16–27. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Liang T, Wen Q, Lin X, Tang J, Zuo Q, Tao L, Xuan F, Huang R. Protective effects of total extracts of Averrhoa carambola L. (Oxalidaceae) roots on streptozotocin-induced diabetic mice. Cell Physiol Biochem. 2014;33(5):1272–82. [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Wang X, Fang X, Lian F, Li M, Liao J, Dai D, Tian J. Metformin modulates the gut microbiome in a mice model of high-fat diet-induced glycolipid metabolism disorder. BMJ Open Diabetes Res Care. 2022;10(6): e003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong W, Feng J, Liu Y, Liu J, Fu L, Wang Q, Li X, Li S. ShenQiWan ameliorates renal injury in type 2 diabetic mice by modulating mitochondrial fusion and Endoplasmic reticulum stress. Front Pharmacol. 2023;14:1265551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YZ, Gu J, Chuang WT, Du YF, Zhang L, Lu ML, Xu JY, Li HQ, Liu Y, Feng HT, Li YH, Qin LQ. Slowly digestible carbohydrate diet ameliorates hyperglycemia and hyperlipidemia in high-fat diet/Streptozocin-induced diabetic mice. Front Nutr. 2022;9: 854725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wick MR. The hematoxylin and eosin stain in anatomic pathology-an often-neglected focus of quality assurance in the laboratory. Semin Diagn Pathol. 2019;36(5):303–11. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Gao Y, Li M, Dong Y, Chen J, Zhang B, Li K, Cai Y. Cai’s herbal tea enhances mitochondrial autophagy of type 1 diabetic mellitus β cells through the ampk/mtor pathway and alleviates inflammatory response. Acta Diabetol. 2024;61(12):1553–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu C, Yang B, Liu Y, Liu W, Yan J, Guo C, Song T, Wang X. Carvacrol regulates the expression of SLC25A6 by inhibiting VDAC1 to improve mitochondrial function and reduce LPS-Induced inflammatory injury in HMEC-1 cells. ACS Omega. 2025;10(8):8512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oselusi SO, Egieyeh SA, Christoffels A. Cheminformatic profiling and hit prioritization of natural products with activities against methicillin-resistant Staphylococcus aureus (MRSA). Molecules. 2021;26(12):3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z, Li W, Liu G, Tang Y. AdmetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics. 2019;35(6):1067–9. [DOI] [PubMed] [Google Scholar]
- 37.Oselusi SO, Christoffels A, Egieyeh SA. Cheminformatic characterization of natural antimicrobial products for the development of new lead compounds. Molecules. 2021;26(13):3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Qu B, Yang D, Wang Y, Zhu H, Wang Z, Zhang X, Ma H, Zhao N, Zhao L, Zhou L, He X, Li P. Combined metabolomics and network pharmacology to elucidate the mechanisms of Huiyang Shengji Decoction in treating diabetic skin ulcer mice. Phytomedicine. 2025;141:156569. [DOI] [PubMed] [Google Scholar]
- 39.Salame G, Hakim V, Dagher C, Daou RM, Dada AE, Nassif L, Ghadieh HE, Azar S, Bazzi S, Harb F. Immunotherapy as a treatment for type 1 diabetes mellitus in children and young adults: A comprehensive systematic review and meta-analysis. PLoS ONE. 2025;20(4):e0321727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altobaishat O, Gadelmawla AF, Almohtasib S, Suilik HA, Manasrah A, Abouzid M, Turkmani M, Abuelazm M. Once-Weekly insulin versus Once-Daily insulin for type 1 diabetes treatment: A systematic review and Meta-Analysis of randomised controlled trials. Endocrinol Diabetes Metab. 2025;8(3):e70048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Y, Jin Y, Li S, Shen B, Ma L, Zuo L, Gao Y, Yang G. Leonurine alleviates cognitive dysfunction and reduces oxidative stress by activating Nrf-2 pathway in alzheimer’s disease mouse model. Neuropsychiatr Dis Treat. 2023;19:1347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu C, Guo W, Maerz S, Gu X, Zhu Y. 3,5-Dimethoxy-4-(3-(2-carbonyl-ethyldisulfanyl)-propionyl)-benzoic acid 4-guanidino-butyl ester: a novel twin drug that prevents primary cardiac myocytes from hypoxia-induced apoptosis. Eur J Pharmacol. 2013;700(1–3):118–26. [DOI] [PubMed] [Google Scholar]
- 43.Shi XR, Hong ZY, Liu HR, Zhang YC, Zhu YZ. Neuroprotective effects of SCM198 on 6-hydroxydopamine-induced behavioral deficit in rats and cytotoxicity in neuronal SH-SY5Y cells. Neurochem Int. 2011;58(8):851–60. [DOI] [PubMed] [Google Scholar]
- 44.Shinde SS, Giram PS, Wakte PS, Bhusari SS. Admet tools in the digital era: applications and limitations. Adv Pharmacol. 2025;103:65–80. [DOI] [PubMed] [Google Scholar]
- 45.Das IJ, Bhatta K, Sarangi I, Samal HB. Innovative computational approaches in drug discovery and design. Adv Pharmacol. 2025;103:1–22. [DOI] [PubMed] [Google Scholar]
- 46.Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc. 2021;1(4): e78. [DOI] [PubMed] [Google Scholar]
- 47.Nelios G, Prapa I, Mitropoulou G, Kompoura V, Balafas E, Kostomitsopoulos N, Yanni AE, Kourkoutas Y. Assessment of immobilized Lacticaseibacillus rhamnosus OLXAL-1 cells on oat flakes for functional regulation of the intestinal Microbiome in a Type-1 diabetic animal model. Foods. 2024;13(24):4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes. 2015;8:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Zhang M, Deng D, Zhu X. The function, mechanisms, and clinical applications of metformin: potential drug, unlimited potentials. Arch Pharm Res. 2023;46(5):389–407. [DOI] [PubMed] [Google Scholar]
- 50.Alam S, Khan SJ, Lee CYF, Zaidi SAT, Murtaza SF. Type 1 diabetes mellitus management and islet cell therapy: a new chapter in patient care. Cureus. 2023;15(10):e46912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang Y, He P, Sheng K, Peng Y, Wu H, Qian S, Ji W, Guo X, Shan X. The protective roles of Eugenol on type 1 diabetes mellitus through NRF2-mediated oxidative stress pathway. Elife. 2025;13:RP96600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Yu H, He J. Role of dyslipidemia in accelerating inflammation, autoimmunity, and atherosclerosis in systemic lupus erythematosus and other autoimmune diseases. Discov Med. 2020;30(159):49–56. [PubMed] [Google Scholar]
- 53.Song C, Zheng W, Song C, Zhou H, Yao J. Protective effects of Food-Derived Kaempferol on pancreatic β-Cells in type 1 diabetes mellitus. Foods. 2024;13(23):3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang X, Yan X, Zhou H, Huang G, Niu X, Jiang H, Su H, Yang X, Li X, Zhou Z. Associations of insulin resistance and beta-cell function with abnormal lipid profile in newly diagnosed diabetes. Chin Med J (Engl). 2022;135(21):2554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Liu C, Xu Y, Wang Y, Dai F, Hu H, Jiang T, Lu Y, Zhang Q. The management correlation between metabolic index, cardiovascular health, and diabetes combined with cardiovascular disease. Front Endocrinol (Lausanne). 2023;13:1036146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karamanakos G, Kokkinos A, Dalamaga M, Liatis S. Highlighting the role of obesity and insulin resistance in type 1 diabetes and its associated cardiometabolic complications. Curr Obes Rep. 2022;11(3):180–202. [DOI] [PubMed] [Google Scholar]
- 57.Dakroub A, Dbouk A, Asfour A, Nasser SA, El-Yazbi AF, Sahebkar A, Eid AA, Iratni R, Eid AH. C-peptide in diabetes: A player in a dual hormone disorder? J Cell Physiol. 2024;239(5):e31212. [DOI] [PubMed] [Google Scholar]
- 58.Dalle S, Abderrahmani A. Receptors and signaling pathways controlling beta-cell function and survival as targets for anti-diabetic therapeutic strategies. Cells. 2024;13(15):1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rafaqat S, Sattar A, Khalid A, Rafaqat S. Role of liver parameters in diabetes mellitus - a narrative review. Endocr Regul. 2023;57(1):200–20. [DOI] [PubMed] [Google Scholar]
- 60.Blagov AV, Summerhill VI, Sukhorukov VN, Popov MA, Grechko AV, Orekhov AN. Type 1 diabetes mellitus: inflammation, mitophagy, and mitochondrial function. Mitochondrion. 2023;72:11–21. [DOI] [PubMed] [Google Scholar]
- 61.Rivera Nieves AM, Wauford BM, Fu A. Mitochondrial bioenergetics, metabolism, and beyond in pancreatic β-cells and diabetes. Front Mol Biosci. 2024;11:1354199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meeprom A, Chan CB, Sompong W, Adisakwattana S. Isoferulic acid attenuates methylglyoxal-induced apoptosis in INS-1 rat pancreatic β-cell through mitochondrial survival pathways and increasing glyoxalase-1 activity. Biomed Pharmacother. 2018;101:777–85. [DOI] [PubMed] [Google Scholar]
- 63.Tomita T. Apoptosis of pancreatic β-cells in type 1 diabetes. Bosn J Basic Med Sci. 2017;17(3):183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson G, Type I, Diabetes Pathoetiology, Pathophysiology. Roles of the gut microbiome, pancreatic cellular interactions, and the ‘bystander’ activation of memory CD8 T cells. Int J Mol Sci. 2023;24(4):3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin W, Lei Y. Leonurine inhibits IL-1β induced inflammation in murine chondrocytes and ameliorates murine osteoarthritis. Int Immunopharmacol. 2018;65:50–9. [DOI] [PubMed] [Google Scholar]
- 67.El-Kersh AOFO, El-Akabawy G, Al-Serwi RH. Transplantation of human dental pulp stem cells in streptozotocin-induced diabetic rats. Anat Sci Int. 2020;95(4):523–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are publicly available from the following databases and repositories: the Lipinski rule of five (http://www.scfbio-iitd.res.in/software/drugdesign/lipinski.jsp), the admetSAR (http://lmmd.ecust.edu.cn/admetsar2/), the Swiss ADME online analyzer (http://www.swissadme.ch/), the TCMSP (http://www.chemsrc.com), the PubChem (https://pubchem.ncbi.nlm.nih.gov/), the PharmMapper (http://www.lilab-ecust.cn), the TargetNet (http://targetnet.scbdd.com/), the Swiss Target Pre-diction (http://www.swisstargetprediction.ch/), the TTD (https://db.idrblab.net/ttd/), the GeneCards (https://www.genecards.org/), the Dis OMIM (https://www.omim.org/), the TCMSP database (https://old. tcmsp-e.com/tcmsp.php), the STRING database (https://string-db.org/), the DAVID database (https://davidbioinformatics.nih.gov/), the Protein Data Bank (www.rcsb.org), the PubChem (https://pubchem.ncbi.nlm.nih.gov/), and the DoGSiteScorer (https://proteins.plus/). The data for this study are included within the manuscript. Additional data are available from the corresponding author upon request.