Abstract
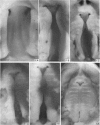
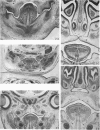
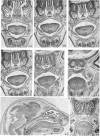
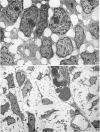
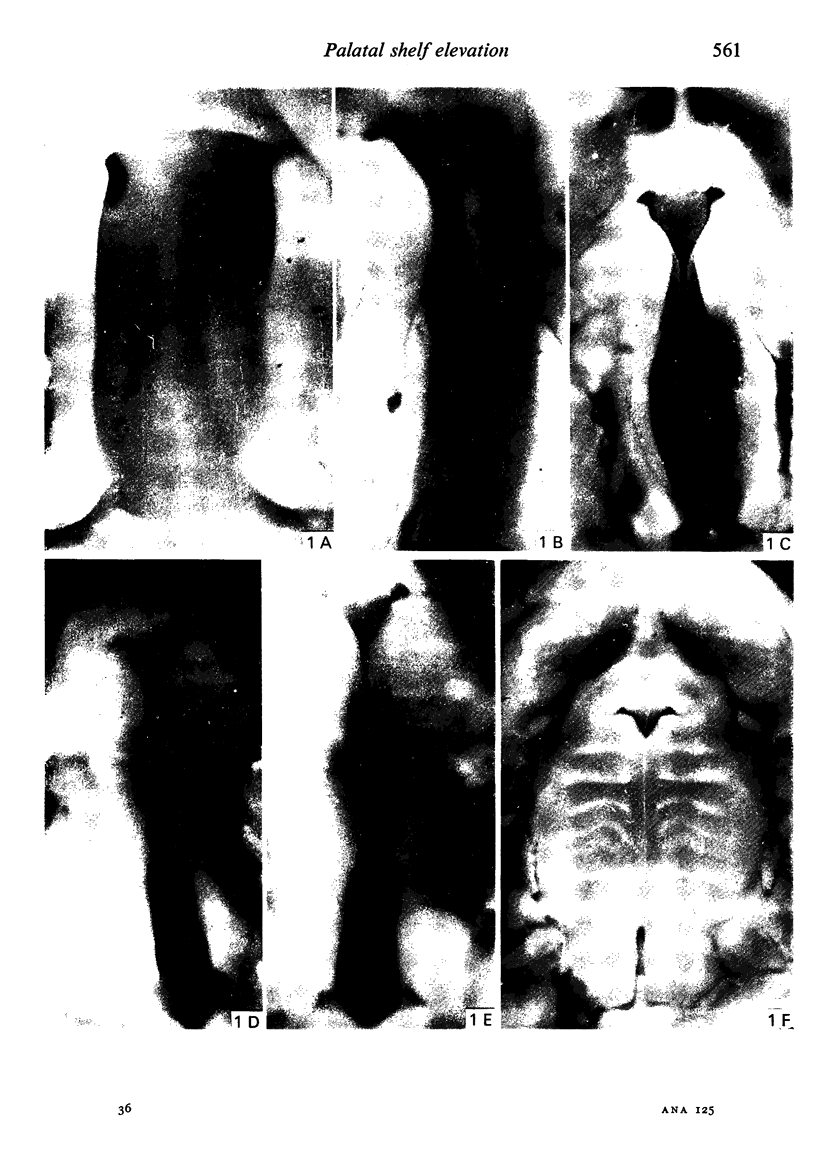
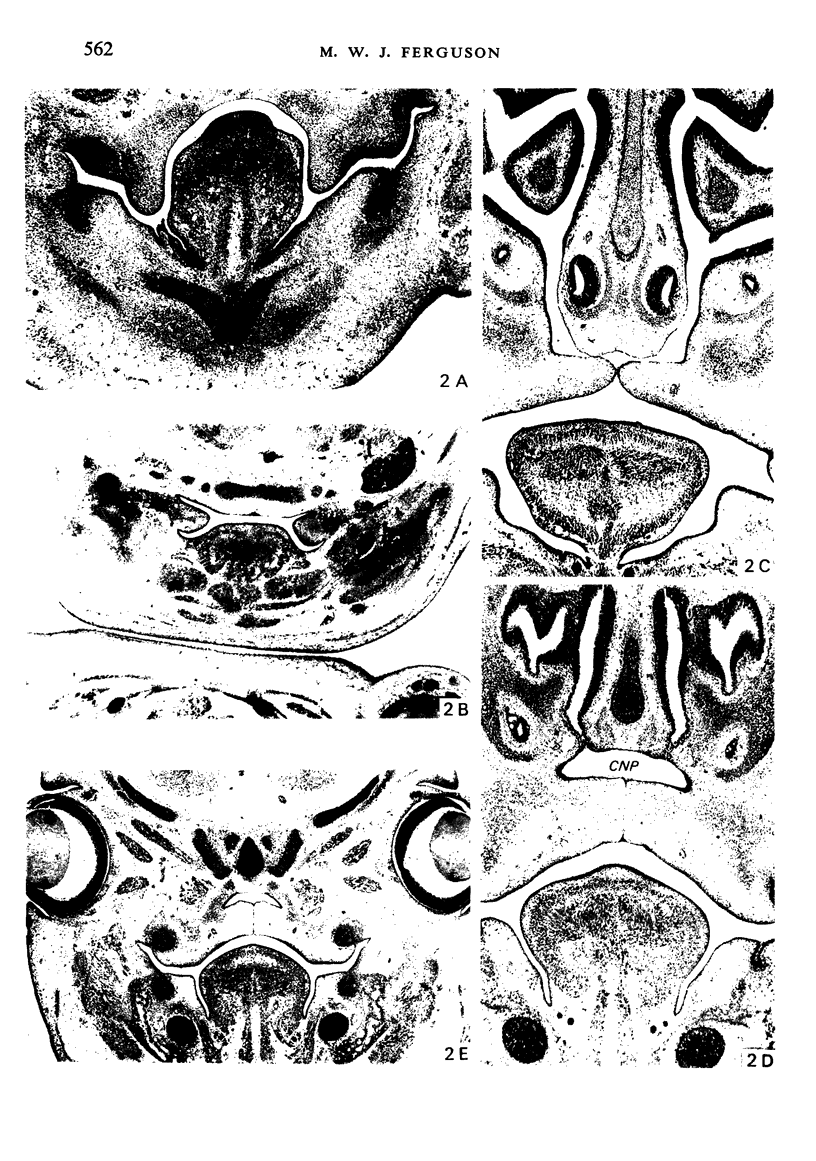
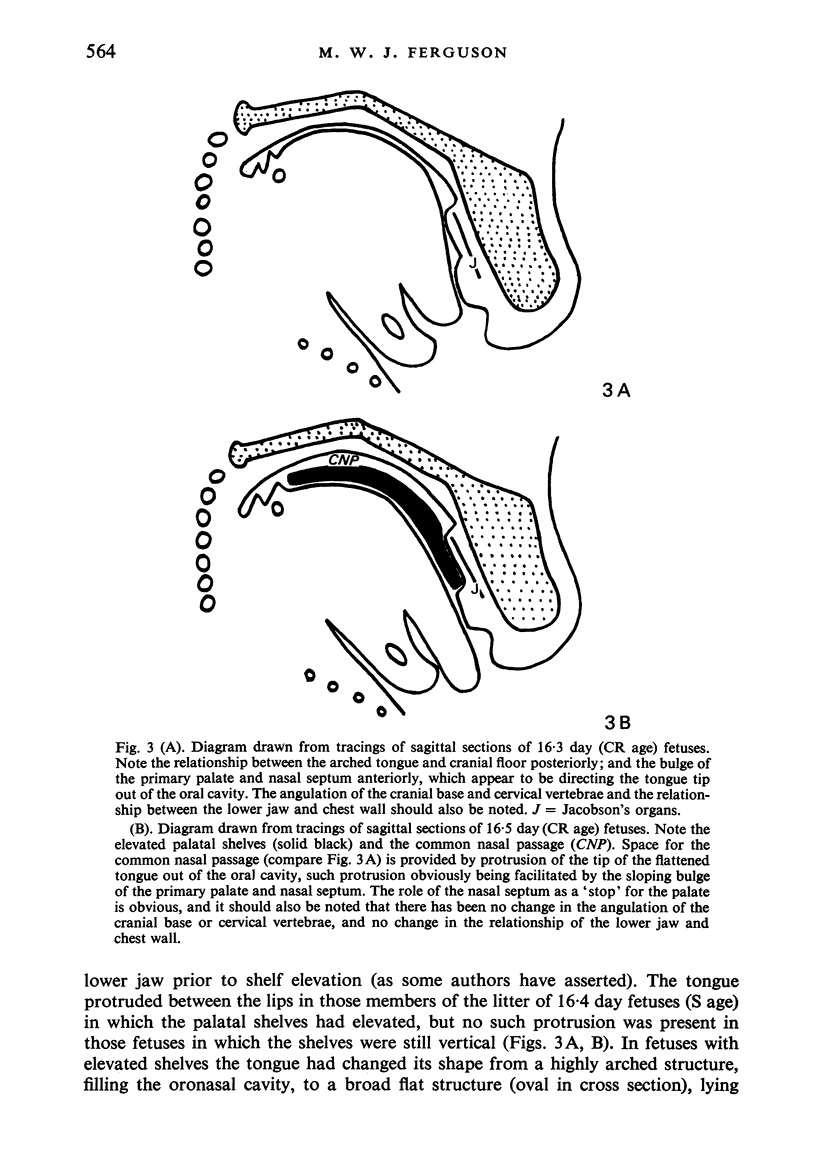
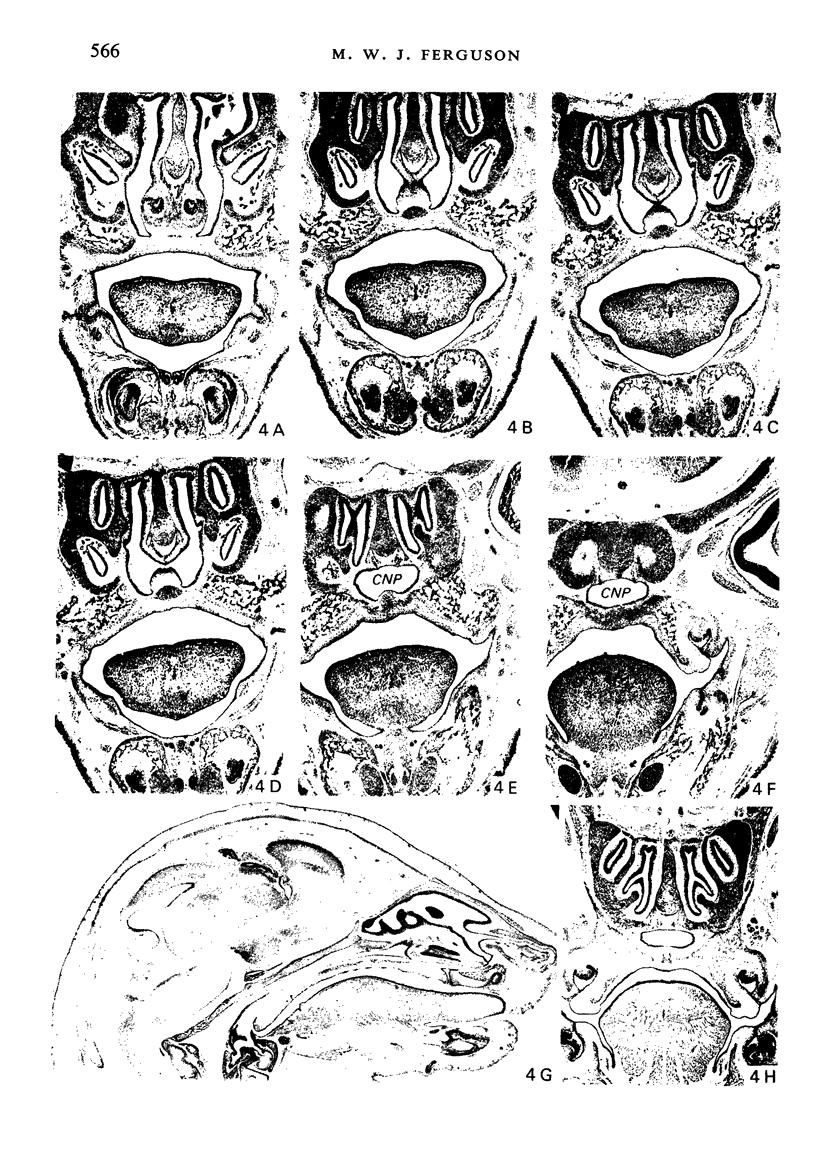
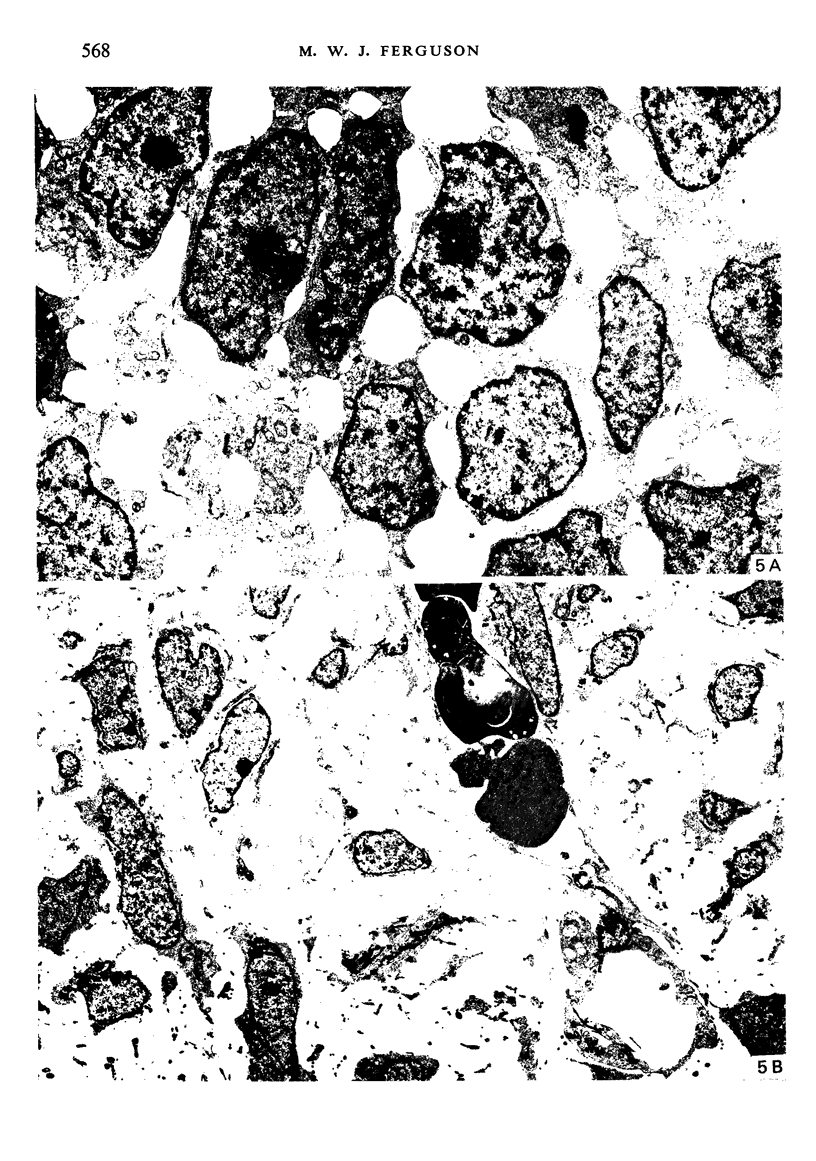
Palatogenesis in the Wistar rat fetus was studied macroscopically, microscopically, ultrastructurally and experimentally between days 13 and 19. The developmental ages of the fetuses were calculated from the smear age of the litter adjusted for individual variations in crown-rump lengths. Palatal shelf elevation occurs at day 16.4 +/- 0.1. Experimentally induced shelf elevation in freshly delivered fetuses was sluggish at day 14, but by day 16.3 it occurred in less than 1 second. Both shelf elevation and shelf fusion begin anteriorly where the shelves show a marked convexity of their margins, and proceed posteriorly. The extreme posterior part of each shelf (future soft palate) is horizontal from the beginning. The matrix of the shelf mesenchyme (especially in the region of the anterior convexities) shows an increasing accumulation of mucopolysaccharides from day 14 to day 16.3 and becomes increasingly oedematous. The shelf attachment to the main maxillary process is progressively undercut by epithelial invagination, producing a fulcrum for shelf elevation. The maxillary and palatine osteogenic blastemata are present at the base of the shelf prior to elevation and rapidly invade the shelves after the event. The elevated palatal shelves fuse with the nasal septum anteriorly, but posteriorly the palate is not attached to the septum. The posterior septum at first has a free lower edge, but then it develops lateral flanges which fuse with corresponding bulges on the lateral nasal walls. In this way two sphenoethmoidal recesses are formed above the fused flanges, while a common nasal passage is formed above the palate, roofed anteriorly by the septal flanges and posteriorly by the cranial base. The space needed to create (simultaneous with shelf elevation) the common nasal passage is made available by flattening of the tongue and protrusion of its tip out of the oral cavity--this protrusion being facilitated by the sloping bulge of the primary palate and nasal septum. Many existing theories of shelf elevation are inconsistent with these observations. It was concluded that shelf elevation occurs very rapidly at a rather precise developmental stage and that turgor (due to binding of water to mucopolysaccharides) is the intrinsic force which elevates the shelves, a force which at 16.4 days reaches a threshold level enabling the shelves to force their way up and over the intervening tongue.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASLING C. W., NELSON M. M., DOUGHERTY H. D., WRIGHT H. V., EVANS H. M. The development of cleft palate resulting from maternal pteroylglutamic (folic) acid deficiency during the latter half of gestation in rats. Surg Gynecol Obstet. 1960 Jul;111:19–28. [PubMed] [Google Scholar]
- Abramovich A. Cleft palate in the fetuses of lathyric rats and its relation to other structures: nasal septum, tongue and mandible. Cleft Palate J. 1972 Jan;9(1):73–83. [PubMed] [Google Scholar]
- Andersen H., Matthiessen M. Histochemistry of the early development of the human central face and nasal cavity with special reference to the movements and fusion of the palatine processes. Acta Anat (Basel) 1967;68(4):473–508. doi: 10.1159/000143050. [DOI] [PubMed] [Google Scholar]
- Andrew F. D., Zimmerman E. F. Glucocorticoid induction of cleft palate in mice; no correlation with inhibition of mucopolysaccharide synthesis. Teratology. 1971 Feb;4(1):31–37. doi: 10.1002/tera.1420040106. [DOI] [PubMed] [Google Scholar]
- Babiarz B. S., Allenspach A. L., Zimmerman E. F. Ultrastructural evidence of contractile systems in mouse palates prior to rotation. Dev Biol. 1975 Nov;47(1):32–44. doi: 10.1016/0012-1606(75)90261-4. [DOI] [PubMed] [Google Scholar]
- Burdi A. R., Silvey R. G. The relation of sex-associated facial profile reversal and stages of human palatal closure. Teratology. 1969 Nov;2(4):297–303. doi: 10.1002/tera.1420020404. [DOI] [PubMed] [Google Scholar]
- COLEMAN R. D. DEVELOPMENT OF THE RAT PALATE. Anat Rec. 1965 Feb;151:107–117. doi: 10.1002/ar.1091510202. [DOI] [PubMed] [Google Scholar]
- Diewert V. M. A cephalometric study of orofacial structures during secondary palate closure in the rat. Arch Oral Biol. 1974 Apr;19(4):303–315. doi: 10.1016/0003-9969(74)90192-7. [DOI] [PubMed] [Google Scholar]
- Fraser F. C. Cleft lip and cleft palate. Science. 1967 Dec 22;158(3808):1603–1606. doi: 10.1126/science.158.3808.1603. [DOI] [PubMed] [Google Scholar]
- Furstman L., Bernick S., Mahan P. Z. The role of the nasal septum in the development of the secondary palate of the rat. Am J Orthod. 1971 Sep;60(3):244–256. doi: 10.1016/0002-9416(71)90133-3. [DOI] [PubMed] [Google Scholar]
- Greene R. M., Kochhar D. M. Palatal closure in the mouse as demonstrated in frozen sections. Am J Anat. 1973 Aug;137(4):477–482. doi: 10.1002/aja.1001370409. [DOI] [PubMed] [Google Scholar]
- Greene R. M., Pratt R. M. Developmental aspects of secondary palate formation. J Embryol Exp Morphol. 1976 Oct;36(2):225–245. [PubMed] [Google Scholar]
- Gregg J. M., Avery J. K. Experimental studies of vascular development in normal and cleft palate mouse embryos. Cleft Palate J. 1971 Apr;8:101–117. [PubMed] [Google Scholar]
- HEDBLOM E. E. The role of polysaccharides in corneal swelling. Exp Eye Res. 1961 Sep;1:81–91. doi: 10.1016/s0014-4835(61)80012-2. [DOI] [PubMed] [Google Scholar]
- Hassel J. R., Orkin R. W. Synthesis and distribution of collagen in the rat palate during shelf elevation. Dev Biol. 1976 Mar;49(1):80–88. doi: 10.1016/0012-1606(76)90259-1. [DOI] [PubMed] [Google Scholar]
- Holt T. M. A morphological and histochemical study of the developing tongue musculature in the mouse: its relationship to palatal closure. Am J Anat. 1975 Oct;144(2):169–195. doi: 10.1002/aja.1001440205. [DOI] [PubMed] [Google Scholar]
- Humphrey T. Development of oral and facial motor mechanisms in human fetuses and their relation to craniofacial growth. J Dent Res. 1971 Nov-Dec;50(6):1428–1441. doi: 10.1177/00220345710500061301. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The relation between human fetal mouth opening reflexes and closure of the palate. Am J Anat. 1969 Jul;125(3):317–344. doi: 10.1002/aja.1001250305. [DOI] [PubMed] [Google Scholar]
- Jacobs R. M. Failure of muscle relaxants to produce cleft palate in mice. Teratology. 1971 Feb;4(1):25–29. doi: 10.1002/tera.1420040105. [DOI] [PubMed] [Google Scholar]
- Jacobs R. M. Normal closure of secondary palate in paralyzed mouse embryos. J Dent Res. 1970 Nov-Dec;49(6 Suppl):1495–1497. doi: 10.1177/00220345700490065601. [DOI] [PubMed] [Google Scholar]
- Johnston M. C., Hassell J. R., Brown K. S. The embryology of cleft lip and cleft palate. Clin Plast Surg. 1975 Apr;2(2):195–203. [PubMed] [Google Scholar]
- Joodeph D. R., Wragg L. E. Facial growth during the secondary palate closure in the rat. Am J Orthod. 1971 Jul;60(1):88–89. doi: 10.1016/0002-9416(71)90190-4. [DOI] [PubMed] [Google Scholar]
- LARSSON K. S. Studies on the closure of the secondary palate. II. Occurrence of sulpho-mucopolysaccharides in the palatine processes of the normal mouse embryo. Exp Cell Res. 1960 Dec;21:498–503. doi: 10.1016/0014-4827(60)90282-2. [DOI] [PubMed] [Google Scholar]
- Lessard J. L., Wee E. L., Zimmerman E. F. Presence of contractile proteins in mouse fetal palate prior to shelf elevation. Teratology. 1974 Feb;9(1):113–125. doi: 10.1002/tera.1420090114. [DOI] [PubMed] [Google Scholar]
- Nanda R. The role of sulfated mucopolysaccharides in cleft palate production. Teratology. 1970 Aug;3(3):237–244. doi: 10.1002/tera.1420030305. [DOI] [PubMed] [Google Scholar]
- Pratt R. M., Goggins J. F., Wilk A. L., King C. T. Acid mucopolysaccharide synthesis in the secondary palate of the developing rat at the time of rotation and fusion. Dev Biol. 1973 May;32(1):230–237. doi: 10.1016/0012-1606(73)90237-6. [DOI] [PubMed] [Google Scholar]
- Pratt R. M., Jr, King C. T. Inhibition of collagen cross-linking associated with beta-aminopropionitrile-induced cleft palate in the rat. Dev Biol. 1972 Mar;27(3):322–328. doi: 10.1016/0012-1606(72)90171-6. [DOI] [PubMed] [Google Scholar]
- ROSS R. B., LINDSAY W. K. THE CERVICAL VERTEBRAE AS A FACTOR IN ETIOLOGY OF CLEFT PALATE. Cleft Palate J. 1965 Jul;36:273–281. [PubMed] [Google Scholar]
- Stark R. B. Development of the face. Surg Gynecol Obstet. 1973 Sep;137(3):403–408. [PubMed] [Google Scholar]
- Verrusio A. C. A mechanism for closure of the secondary palate. Teratology. 1970 Feb;3(1):17–20. doi: 10.1002/tera.1420030105. [DOI] [PubMed] [Google Scholar]
- WALKER B. E. The association of mucopolysaccharides with morphogenesis of the palate and other structures in mouse embryos. J Embryol Exp Morphol. 1961 Mar;9:22–31. [PubMed] [Google Scholar]
- WOOD P. J., KRAUS B. S. Prenatal development of the human palate. Some histological observations. Arch Oral Biol. 1962 Mar-Apr;7:137–150. doi: 10.1016/0003-9969(62)90002-x. [DOI] [PubMed] [Google Scholar]
- Walker B. E. Correlation of embryonic movement with palate closure in mice. Teratology. 1969 Aug;2(3):191–197. doi: 10.1002/tera.1420020304. [DOI] [PubMed] [Google Scholar]
- Walker B. E. Palate morphogenesis in the rabbit. Arch Oral Biol. 1971 Mar;16(3):275–286. doi: 10.1016/0003-9969(71)90021-5. [DOI] [PubMed] [Google Scholar]
- Walker B. E., Patterson A. Induction of cleft palate in mice by tranquilizers and barbiturates. Teratology. 1974 Oct;10(2):159–163. doi: 10.1002/tera.1420100212. [DOI] [PubMed] [Google Scholar]
- Wragg L. E., Diewert V. M., Klein M. Spatial relations in the oral cavity and the mechanism of secondary palate closure in the rat. Arch Oral Biol. 1972 Apr;17(4):683–690. doi: 10.1016/0003-9969(72)90194-x. [DOI] [PubMed] [Google Scholar]
- Wragg L. E., Klein M., Steinvorth G., Warpeha R. Facial growth accommodating secondary palate closure in rat and man. Arch Oral Biol. 1970 Aug;15(8):705–719. doi: 10.1016/0003-9969(70)90035-x. [DOI] [PubMed] [Google Scholar]
- Wragg L. E., Smith J. A., Borden C. S. Myoneural maturation and function of the foetal rat tongue at the time of secondary palate closure. Arch Oral Biol. 1972 Apr;17(4):673–682. doi: 10.1016/0003-9969(72)90193-8. [DOI] [PubMed] [Google Scholar]
- ZEILER K. B., WEINSTEIN S., GIBSON R. D. A STUDY OF THE MORPHOLOGY AND THE TIME OF CLOSURE OF THE PALATE IN THE ALBINO RAT. Arch Oral Biol. 1964 Sep-Oct;9:545–554. doi: 10.1016/0003-9969(64)90018-4. [DOI] [PubMed] [Google Scholar]