Abstract
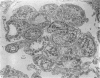
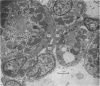
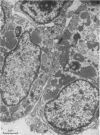
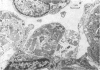
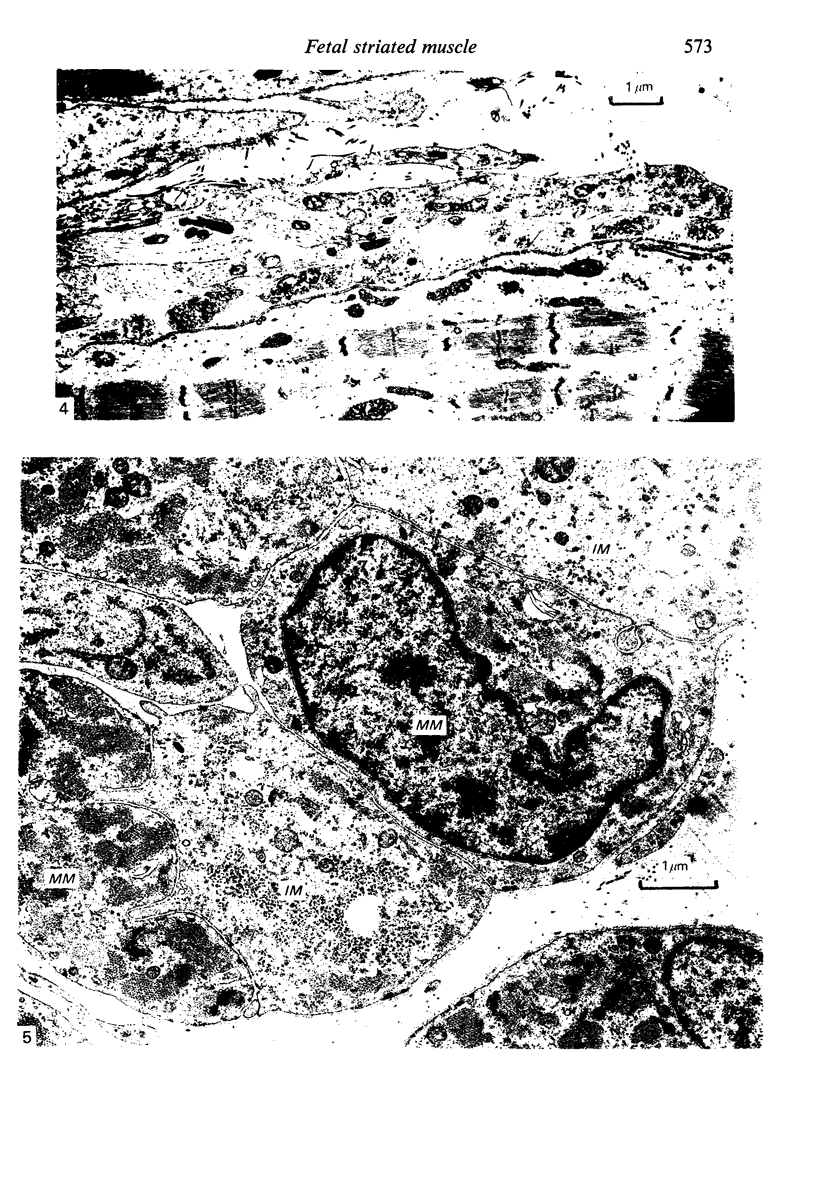
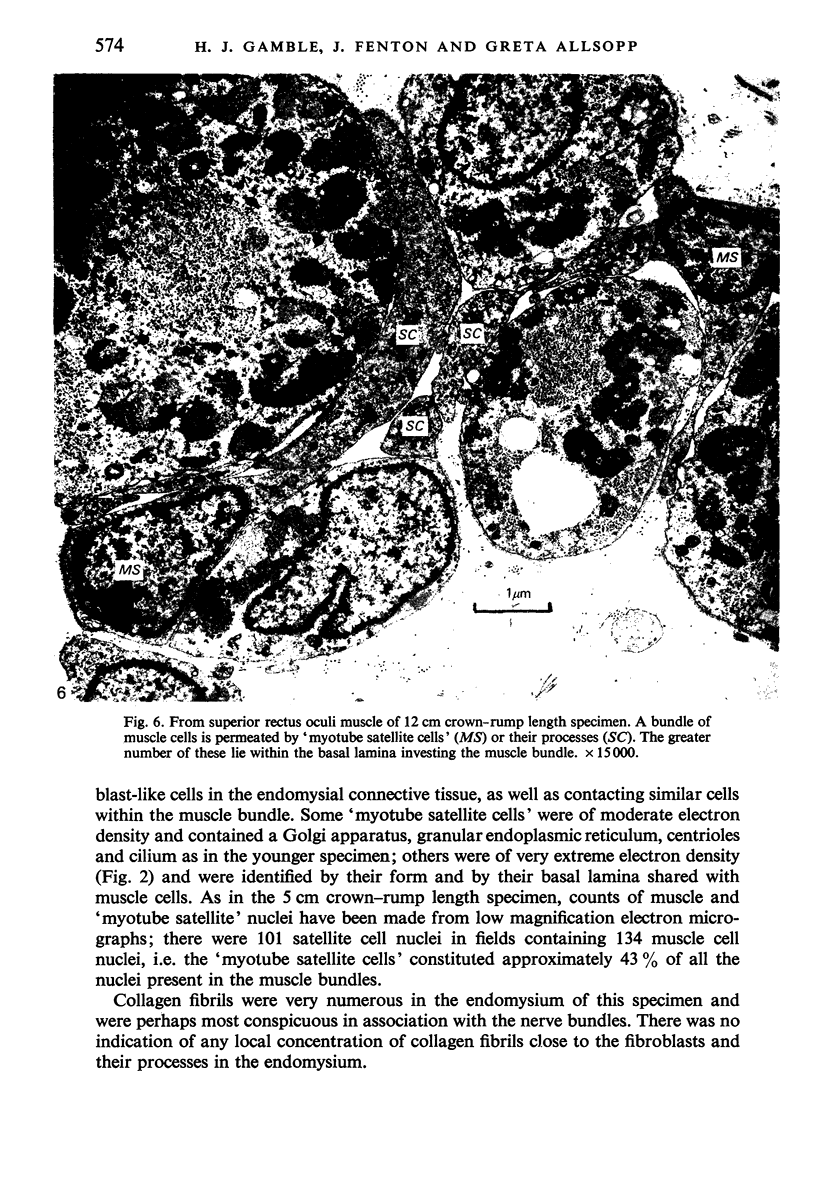
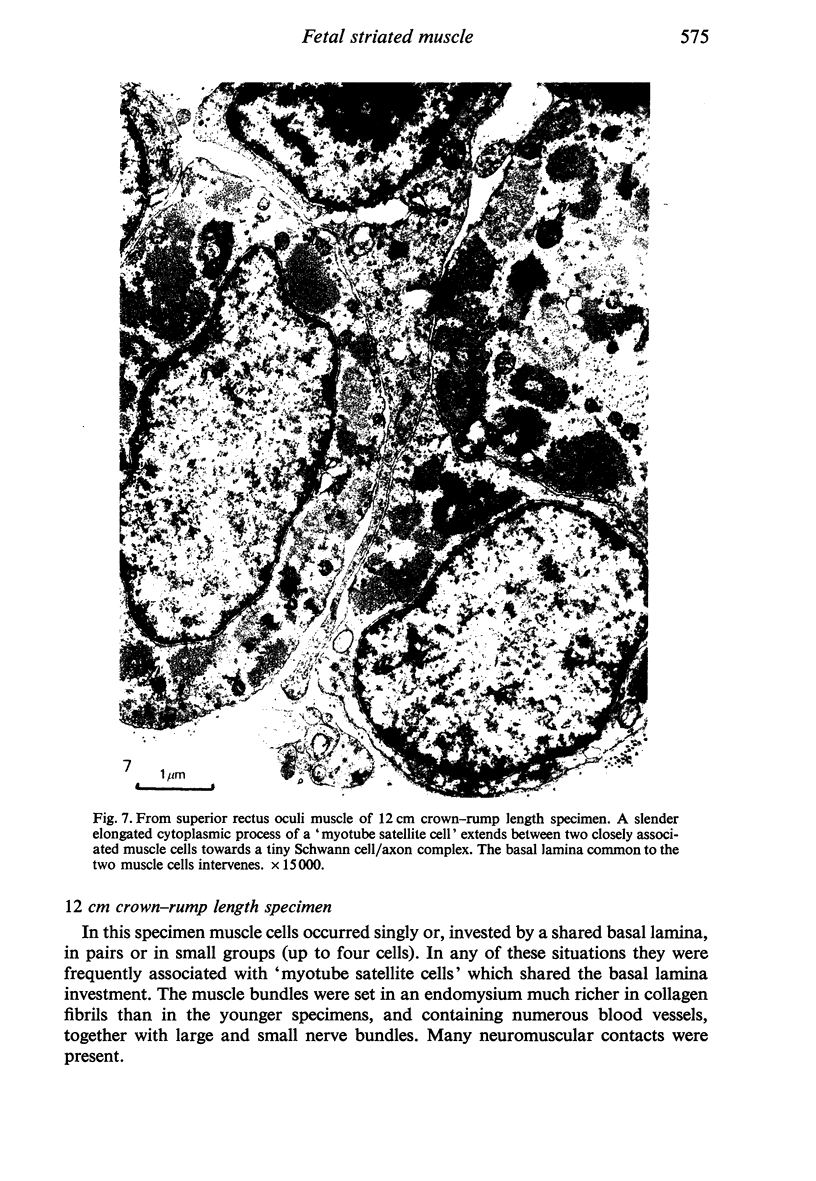
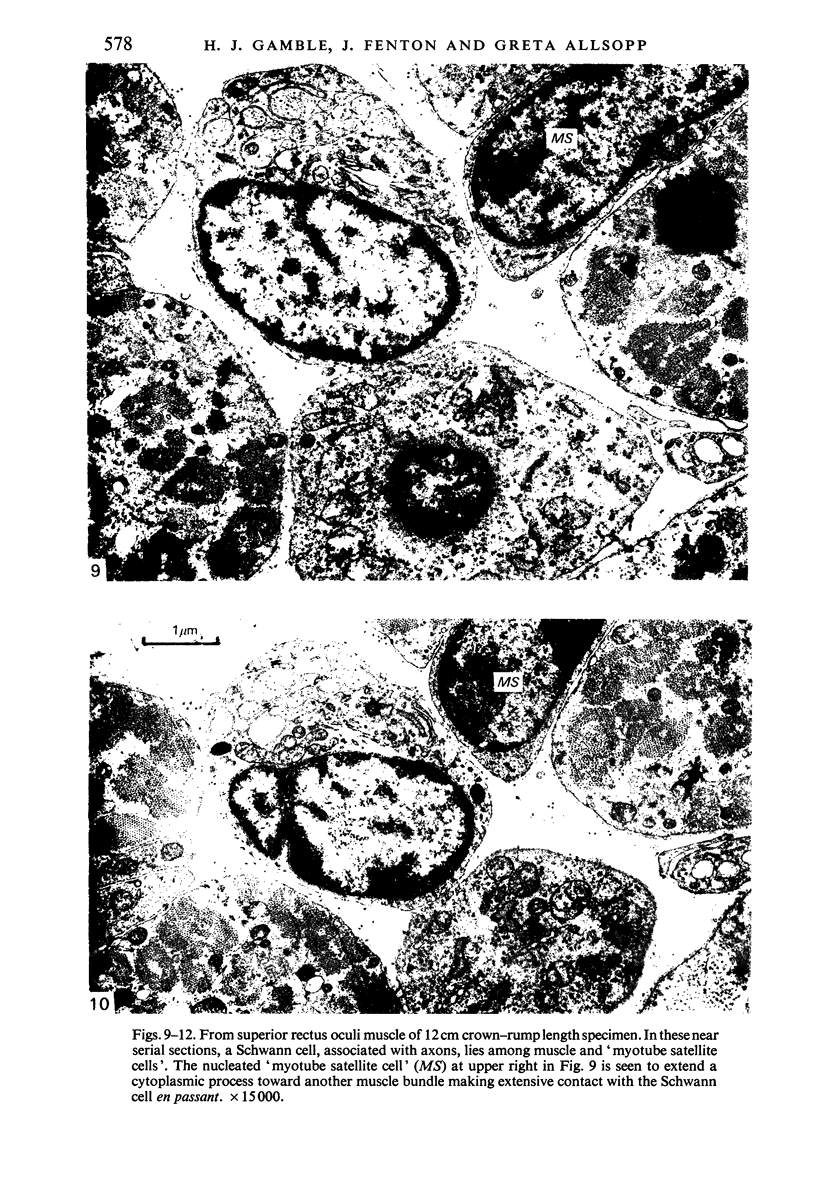
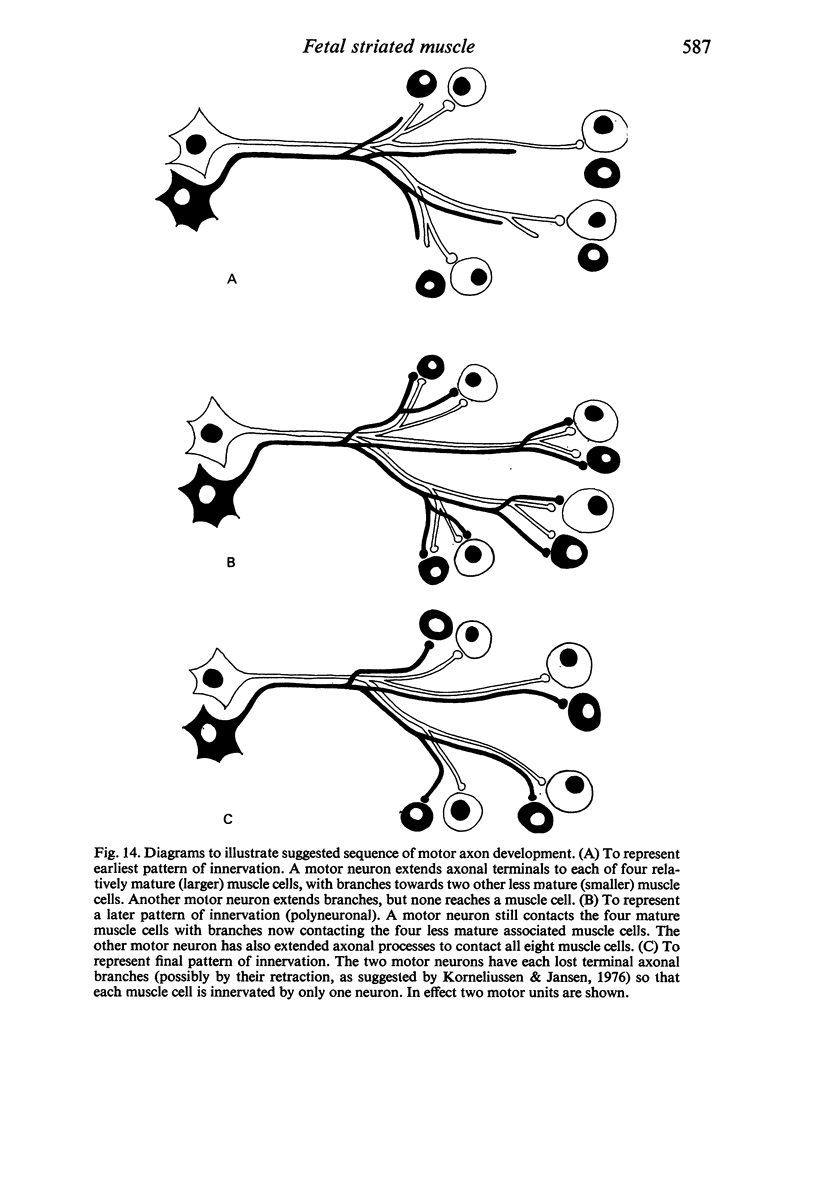
The superior rectus oculi muscle from human fetuses of 5, 9.2, 12 and 24 cm crown--rump length (of ages estimated to be 10, 12, 15 and 23 weeks respectively) have been examined by electron microscopy. "Myotube satellite cells" closely associated with myotubes and myocytes were present in all specimens, but their relative numbers declined with advancing age. Some were small with scanty cytoplasm containing few organelles. Others were rich in organelles, including Golgi apparatus, granular endoplasmic reticulum, comma and dumb-bell shaped dense bodies and centriole or basal body: these cells were numerous in the three smaller specimens but almost absent from the largest. Seemingly active "myotube satellite cells" often extended cytoplasmic processes beyond their confining basal laminae into the endomysial space to contact freelying cells of similar appearance, as well as axon-associated Schwann cells, often to form an extensive network. These "myotube satellite cells" resembled Schwann cells in all respects save association with axons, and it is suggested that they are, indeed, Schwann cells so disposed as to promote axonal growth towards differentiating, but as yet uninnervated, myocytes. Neuromuscular contacts were increasingly numerous with advancing age, usually where several or many axonal terminals contacted a relatively mature (myofilament-rich) muscle cell. Immature myotubes seldom made contact with axonal terminals, even when a closely adjacent (and "coupled") mature muscle cell did so. A sequence of axonal growth and retraction has been proposed which reconciles accounts of early but temporary polyneuronal innervation with commonly accepted ideas regarding the scattered distribution of the muscle components of motor units.
Full text
PDF

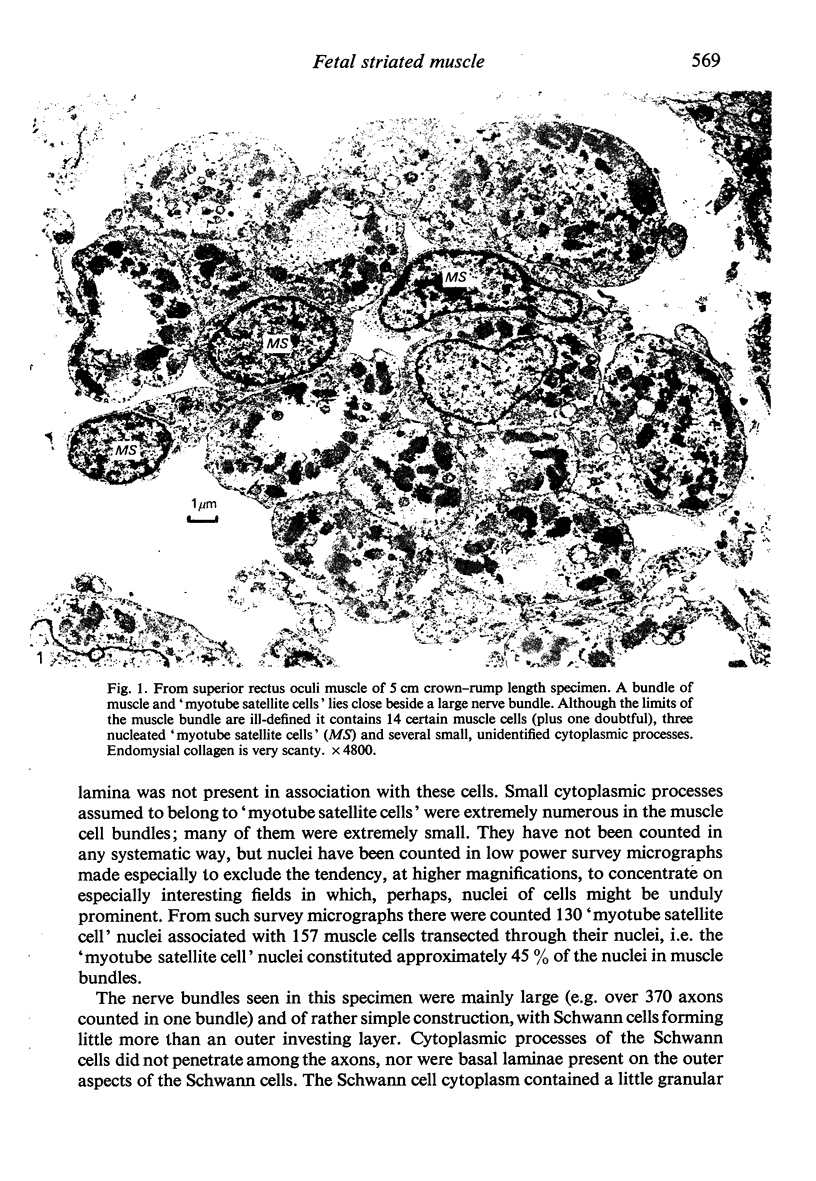













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Minoda K., Davidowitz J., Liebowitz A., Breinin G. M. Fine structure of extraocular muscle in rabbit. J Cell Biol. 1968 Oct;39(1):193–197. doi: 10.1083/jcb.39.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J. C. Satellite cells and myogenesis; a study in the fruit-bat web. J Anat. 1969 Nov;105(Pt 3):419–438. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P. A numerical study of cervical and thoracic ventral nerve roots. J Anat. 1974 Sep;118(Pt 1):127–142. [PMC free article] [PubMed] [Google Scholar]
- HUGHES A. The development of the peripheral nerve fibre. Biol Rev Camb Philos Soc. 1960 Aug;35:283–323. doi: 10.1111/j.1469-185x.1960.tb01326.x. [DOI] [PubMed] [Google Scholar]
- Hewer E. E. The Development of Nerve Endings in the Human Foetus. J Anat. 1935 Apr;69(Pt 3):369–379. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H. Electron microscopic observations of satellite cells with special reference to the development of mammalian skeletal muscles. Z Anat Entwicklungsgesch. 1966;125(1):43–63. doi: 10.1007/BF00521974. [DOI] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The fine structure of motor endplate morphogenesis. J Cell Biol. 1969 Jul;42(1):154–169. doi: 10.1083/jcb.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The histogenesis of rat intercostal muscle. J Cell Biol. 1969 Jul;42(1):135–153. doi: 10.1083/jcb.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen H., Jansen J. K. Morphological aspects of the elimination of polyneuronal innervation of skeletal muscle fibres in newborn rats. J Neurocytol. 1976 Oct;5(8):591–604. doi: 10.1007/BF01175572. [DOI] [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A. J., McNeer K. W., Hay S. H., Watson A. Extraocular muscles: morphogenetic study in humans. Light microscopy and ultrastructural features. Acta Neuropathol. 1977 May 16;38(2):87–93. doi: 10.1007/BF00688553. [DOI] [PubMed] [Google Scholar]
- Miller J. E. Cellular organization of rhesus extraocular muscle. Invest Ophthalmol. 1967 Feb;6(1):18–39. [PubMed] [Google Scholar]
- Page S. G. A comparison of the fine structures of frog slow and twitch muscle fibers. J Cell Biol. 1965 Aug;26(2):477–497. doi: 10.1083/jcb.26.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestige M. C. Evidence that at least some of the motor nerve cells that die during development have first made peripheral connections. J Comp Neurol. 1976 Nov 1;170(1):123–133. doi: 10.1002/cne.901700109. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Margreth A. Coordinated development of the sarcoplasmic reticulum and T system during postnatal differentiation of rat skeletal muscle. J Cell Biol. 1969 Jun;41(3):855–875. doi: 10.1083/jcb.41.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]