Abstract
Background
The mitochondrial genome (mitogenome) of plants exhibits remarkable structural complexity and evolutionary plasticity, yet remains poorly characterized in many ornamental species. Hydrangea chinensis Maxim. (H. chinensis), an indigenous plant distributed across China, serves as a vital genetic resource owing to its morphological diversity, ecological adaptability, and utility in hybrid breeding programs. Despite its importance for enhancing hydrangea varieties, no genomic resources have been published for this species to date. To bridge this knowledge gap, we sequenced and assembled the complete mitogenome of H. chinensis, providing novel insights into its structure, evolution, and functional dynamics.
Results
The mitochondrial genome of H. chinensis was successfully assembled using a hybrid sequencing approach integrating long-read and short-read technologies. The mitogenome spans 722,918 base pairs with a GC content of 45.33%, encoding 38 protein-coding genes along with 21 tRNA genes and 3 rRNA genes. Comprehensive analyses revealed extensive structural features including 335 repeat pairs, 211 simple sequence repeats (SSRs), and significant recombination events that contribute to its multi-branched architecture comprising both circular and linear contigs. We identified 613 C-to-U RNA editing sites affecting key mitochondrial genes such as nad4, suggesting functional roles in post-transcriptional regulation. Furthermore, the genome harbors 23 plastid-derived DNA fragments (MTPTs) spanning 11,059 bp—evidence of chloroplast-to-mitochondrial gene transfer with potential evolutionary implications. Phylogenetic analysis based on conserved mitochondrial protein-coding genes positioned H. chinensis within the Cornales order under Hydrangeaceae while revealing extensive collinearity variations among closely related species.
Conclusion
This study represents the inaugural comprehensive investigation of the mitogenome of H. chinensis, unveiling its intricate architecture shaped by repetitive sequences, dynamic recombination events, RNA editing processes, and inter-organelle gene transfers. These findings enhance our understanding of mitochondrial genome evolution and offer essential genetic resources to support future breeding strategies for hydrangea improvement.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-07119-z.
Keywords: Hydrangea chinensis maxim., Mitochondrial genome, RNA editing, Gene transfer, MTPT
Introduction
The Hydrangea chinensis, indigenous to China and Japan, is a close relative of the widely cultivated ornamental plant, the bigleaf hydrangea (Hydrangea macrophylla (Thunb.) Ser.), both belonging to the family Hydrangeaceae. Although H. chinensis is not widely cultivated as an ornamental plant, it plays a crucial role as a parent species in hybrid breeding programs aimed at improving existing hydrangea varieties. This species exhibits a broad distribution across China and demonstrates remarkable morphological diversity and adaptability, making it a valuable genetic resource for breeding efforts. To date, neither the nuclear genome nor the organelle genomes (chloroplast and mitochondrial) of H. chinensis have been sequenced, despite its widespread use in hybridization studies. Previous studies have only focused on the nuclear and mitochondrial genomes of closely related species, such as H. macrophylla [1]. The lack of genomic information for H. chinensis limits our understanding of its evolutionary dynamics and potential contributions to the genetic improvement of cultivated hydrangeas. Beyond its role in breeding, hydrangeas, including H. chinensis, hold substantial importance within the floriculture industry, with applications spanning landscape design, potted plants, and the cut flower markets [2]. Furthermore, owing to its purported properties such as heat-clearing, detoxification, and anti-inflammatory effects [3, 4], H. chinensis finds a place in traditional medicine practices.
Mitochondria serve as the cellular powerhouses and are vital for the life processes of eukaryotic organisms. Although mitochondria possess their own genomes, most mitochondrial proteins are encoded by nuclear DNA [5]. Distinctive from animal mitochondrial genomes, plant mitochondrial genomes exhibit notable variations in size and structure; they tend to be larger and more complex [6]. Typically existing as double-stranded DNA molecules that may be circular or linear depending on species specifics [7], these genomes are characterized by significant amounts of repetitive sequences. Such complexity poses challenges for assembly and sequencing due to frequent recombination events occurring within them [8]. The mutation rate of mitochondrial genomes is relatively high partly because they lack protective histones alongside efficient DNA repair mechanisms [9]. Recent advancements in long-read sequencing technologies like PacBio and Oxford Nanopore have made sequencing plant mitochondrial genomes increasingly feasible and cost-effective [10, 11].
Despite considerable focus on the nuclear genome and chloroplast genome of hydrangeas in recent years, research concerning their mitochondrial genome remains sparse. To date, there has been no systematic investigation into the mitochondrial genomic resources of hydrangeas—an oversight that constrains our understanding of evolutionary dynamics among hydrangea species’ mitochondrial genomes. Homologous recombination is a prevalent phenomenon within plant mitochondria often leading to genomic rearrangements along with duplication or exchange across intergenic regions [12]. This recombination offers novel insights into evolutionary pathways—highlighting genome diversity along with hybrid vigor aspects [13].
RNA editing—particularly C-to-U transitions—is commonly observed within plant mitochondria; this process modifies transcript coding sequences creating novel start or stop codons ultimately affecting protein function via altered amino acid sequences [14, 15]. In hydrangea’s mitochondrial genome contextually speaking RNA editing could play an instrumental role regulating both functional dynamics alongside gene expression levels though current research exploring these aspects remains limited.
Another critical genetic event is cytoplasmic inheritance where chloroplast-derived DNA segments (termed as mitochondrially located plastid DNA segments or MTPTs) integrate into the mitochondrial genome adding layers of structural complexity while hinting at potential mutational activities therein [16, 17]. Additionally cross-organelle DNA transfers unveil intriguing perspectives regarding plant genomic evolution.
In this study we sequenced t nd subsequently assembled the complete mitochondrial genome of H. chinensis. Our objective was not merely to elucidate its complex architecture but also to examine patterns of repetitive sequences and to provide a comprehensive analysis of recombination events throughout the genome. Moreover comparative assessments were conducted against other closely related Hydrangeaceae members focusing particularly upon inter-genomic synteny analyses amongst near-relatives which collectively enriches existing understanding surrounding evolutionary trajectories inherent within these organisms’ organellar compositions whilst furnishing invaluable genetic resources pivotal for future breeding endeavors targeting improved strains thereof [18]. By elucidating structural-functional paradigms intrinsic underlying mechanisms governing Hydrageae’s mitogenomic fabrications we aspire laying solid groundwork indispensable toward fostering continued exploration regarding organelle-centered genomic evolutions catalyzing diversity manifestations consequently impacting hybridization phenomena amidst flora at large thus paving way forward toward uncharted scientific frontiers burgeoning from foundational discoveries herein articulated.
Materials and methods
Plant material, DNA and RNA extraction, and sequencing
Fresh young leaves were collected from the Shuangxi Town in Pingnan County, Fujian Province (E119°03’19’’, N27°03’28”, elevation 924 m) by the Shanghai Chenshan Botanical Garden. The live leaf samples were washed with sterile water and cleaned before being flash-frozen in liquid nitrogen. Subsequently, they were stored at − 80 °C until further use. Total genomic DNA was extracted using the cetyltrimethylammonium bromide (CTAB) method [19], followed by a plant genomic DNA kit provided by Tiangen Biotech Co., Ltd., Beijing, China. The same DNA samples were utilized for sequencing on both the BGI platform and Oxford Nanopore technology. As part of this study, 350 bp fragments were incorporated into DNA libraries for short-read sequencing on the MGI BGI T7 platform. For Oxford Nanopore sequencing, DNA libraries were prepared using a kit with catalogue number SQK-LSK110, and sequencing was conducted by Wuhan Benagen Technology Co., Ltd.
Mitochondrial genome assembly
To acquire the complete mitochondrial genome sequence of the H. chinensis and explore its potential structural features, this study employed a hybrid assembly method utilizing both long-read data from Oxford Nanopore sequencing and short-read data from BGI sequencing. Initially, assembly was performed directly on the long-read sequencing data using Flye (v2.9.2) software [20] with default parameters, resulting in a graphical assembly output in GFA format. All assembled contigs in FASTA format were used to construct a database with makeblastdb. Subsequently, BLASTn (v2.13.0) was employed to identify contig fragments containing mitochondrial genes using those from Arabidopsis as query sequences, with parameters set at “-evalue 1e-5 -outfmt 6 -max_hsps 10 -word_size 7 -task blastn-short.” The GFA files were visualized using Bandage software (v0.8.1) [21], enabling the screening of mitochondrial contigs based on BLASTn results to obtain a preliminary sketch of the hydrangea’s mitochondrial genome. Next, bwa software (v0.7.17) [22] was utilized to align both long-read and short-read data against the mitochondrial contigs. Aligned mitochondrial reads were filtered and exported for further use in subsequent hybrid assembly processes. Finally, employing a hybrid assembly strategy that integrates both aforementioned sequencing datasets, the hydrangea’s mitochondrial genome was assembled using Unicycler (v0.5.0) [23] with default settings, culminating in the finalized mitochondrial genome sequence. Visualization of this genome was accomplished through Bandage software.
Annotation and codon usage bias analysis of the mitochondrial genome
To achieve a high-quality structural annotation of the mitochondrial genome, we employed Arabidopsis thaliana (NC_037304) and Liriodendron tulipifera (NC_021152.1) as reference genomes. The annotation process was conducted using Geseq software (v2.03) [24]. Additionally, the mitochondrial genome of the species was annotated using the IPMGA tool for angiosperm mitochondrial genomes (http://www.1kmpg.cn/ipmga/), which excels at annotating splice sites and trans-splicing genes. Transfer RNA genes within the mitochondrial genome were annotated with tRNAscan-SE software (v2.0.11) [25], while ribosomal RNA genes were identified using BLASTN software (v2.13.0) [26]. To enhance annotation accuracy, manual corrections and validation of potential annotation errors were performed using Apollo software (v1.11.8) [27].
For analyzing codon usage bias in mitochondrial proteins, coding sequences from the genome were first extracted utilizing Phylosuite software (v1.1.16) [28]. Subsequently, Mega software (v7.0) [29] was employed to analyze codon usage bias within protein-coding genes of the mitochondrial genome and calculate Relative Synonymous Codon Usage (RSCU) values.
Analysis of repetitive sequences
The identification of repetitive sequences within the mitochondrial genome was conducted using a suite of computational tools. We utilized MISA (v2.1) (https://webblast.ipk-gatersleben.de/misa/) [30], TRF (Tandem Repeats Finder, v4.09) (https://tandem.bu.edu/trf/trf.unix.help.html) [31], and the REPuter web server (https://bibiserv.cebitec.uni-bielefeld.de/reputer/) [32] to detect various types of repeats, including microsatellite repeats, tandem repeats, and dispersed repeats. The visualization of these results was accomplished using Excel 2021 and the Circos package (v0.69.9) [33].
Predictive analysis of mitochondrial chloroplast DNA
Throughout the course of mitochondrial evolution, segments of chloroplast DNA can occasionally migrate into the mitochondrial genome. The length and sequence similarity of these migrated fragments exhibit variability across different species. In this study, we employed GetOrganelle (v1.7.7.1) software [34] for the assembly of chloroplast genomes and annotated these genomes using CPGAVAS2 (v1.0) [35]. Subsequently, the annotation results were refined with the assistance of CPGView (v1.0) software [36]. A homologous segment analysis was performed using BLASTN, employing parameters set to an e-value threshold of 1e-5 and a minimum percentage identity of 80% (v2.13.0) [26], and data visualization was achieved through the Circos package (v0.69.9) [33]. This approach ultimately enabled us to identify potential migratory sequences between chloroplasts and mitochondria.
Phylogenetic analysis
To elucidate the evolutionary history and phylogenetic position of H. chinensis, we selected 28 species (Hydrangea macrophylla (PP542010.1), Camellia sinensis (NC_043914.1), Camellia gigantocarpa (OP270590.1), Rhododendron decorum (NC_073150.1), Camellia nitidissima (NC_067639.1), Rhododendron × pulchrum (NC_067943.1), Actinidia chinensis (NC_065997.1), Diospyros oleifera (NC_065039.1), Actinidia eriantha (MZ959063.1), Aegiceras corniculatum (NC_056358.1), Rhododendron simsii (NC_053763.1), Vaccinium macrocarpon (NC_023338.1), Pereskia aculeata (NC_067638.1), Suaeda glauca (NC_060419.1), Agrostemma githago (NC_057604.1), Mirabilis jalapa (NC_056991.1), Tetragonia tetragonoides (MW971440.1), Fallopia aubertii (MW664926.1), Bougainvillea spectabilis (NC_056281.1), Mirabilis himalaica (NC_048974.1), Sesuvium portulacastrum (MN683736.1), Chenopodium quinoa (NC_041093.1), Spinacia oleracea (NC_035618.1), Beta macrocarpa (NC_015994.1), Beta vulgaris subsp. maritima (NC_015099.1), Silene latifolia (NC_014487.1), Malania oleifera (NC_053625.1), Tolypanthus maclurei (NC_056836.1)) from four orders within the angiosperms that are closely related. The mitochondrial genomes of these species were downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov/genome/browse/#!/organelles/). Utilizing PhyloSuite software (v1.1.16) [28], we extracted shared genes across these species. Subsequent multiple sequence alignment was conducted with MAFFT software (v7.505) [37]. A phylogenetic tree was then constructed using the maximum likelihood method via IQ-TREE software (v1.6.12) [38], employing the parameters “--alrt 1000 -B 1000” to ensure robustness and reliability in branch support values. The Best-fit substitution model used for ML tree construction was GTR + F + I + R2, which was selected based on model testing implemented in IQ-TREE. The results of this phylogenetic analysis were visualized with ITOL software (v6) [39].
RNA editing site identification
To identify RNA editing sites, the sequences of all protein-coding genes (PCGs) encoded by the mitochondrial genome of the species were used as input files. The tool Deepred-mt (v1.0) [40] was employed to predict C-to-U RNA editing sites within these mitochondrial PCGs. This tool leverages a convolutional neural network (CNN) model for its predictions, offering superior accuracy compared to traditional prediction methodologies. Only results with probability values exceeding 0.9 were retained, ensuring a high level of confidence in the identified editing sites.
Synteny analysis
To identify conserved homologous sequences, known as syntenic blocks, the BLASTn program was utilized with parameters set to ‘-evalue 1e-5, -word_size 9, -gapopen 5, -gapextend 2, -reward 2, -penalty − 3’. Only syntenic blocks exceeding 500 bp in length were selected for subsequent analysis. Employing MCScanX (v1.0) [41], pairwise comparisons were conducted to generate a multiple synteny plot based on the results obtained from the BLASTn analyses. This facilitated the illustration of conserved syntenic regions. Leveraging sequence similarity data, we employed the core functionalities of MCScanX to visualize a multiple synteny plot between H. chinensis and its closely related species, enhancing interpretability through graphical representation.
Result
Assembly of the mitochondrial genome
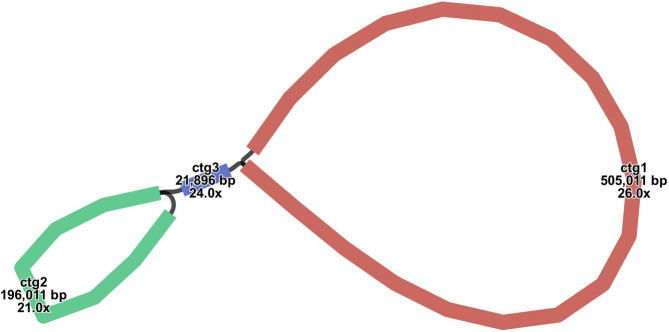
To assemble the mitochondrial genome of H. chinensis, we employed a hybrid assembly approach that integrates both long-read and short-read sequencing data. From the total genomic DNA, we successfully assembled the H. chinensis’s mitochondrial genome. The preliminary draft of this assembly, constructed using long-read data, was visualized with Bandage software. This visualization yielded a graphical representation of the mitochondrial genome structure, as depicted in Fig. 1, revealing an interconnected model comprising three contigs. The principal architecture of the hydrangea mitochondrial genome is characterized by a multi-branched configuration, extending to a total length of 722,918 base pairs (bp) with a GC content of 45.33%. Detailed analysis using long-reads at junctions determined that two of these contigs can form circular structures autonomously, while one remains as an independent linear sequence (Fig. 1). Comprehensive details regarding each assembled node are summarized in Table 1. To assess the reliability of our assembly, the original reads were aligned to the assembled contigs. The coverage results (Fig S1−3) indicated continuity within the genomic sequence, with no gaps detected. This confirms the robustness and reliability of our mitochondrial genome assembly results.
Fig. 1.
Schematic representation of the mitochondrial genome assembly of H. chinensis. This figure presents a visualization of the assembled mitochondrial genome, rendered using Bandage. The assembly reveals three distinct chromosomes, providing insights into the structural organization and genomic architecture of the mitogenome
Table 1.
Summary of basic information on the H. chinensis mitochondrial genome
| Number | Contigs | type | Length (bp) | GC content (%) |
|---|---|---|---|---|
| 1 | Chromosome 1–3 | Branched | 722,918 | 45.33 |
| 2 | Chromosome 1 | Circular | 505,011 | 45.31 |
| 3 | Chromosome 2 | Circular | 196,011 | 45.38 |
| 4 | Chromosome 3 | Linear | 21,896 | 45.58 |
Features of the mitochondrial genome
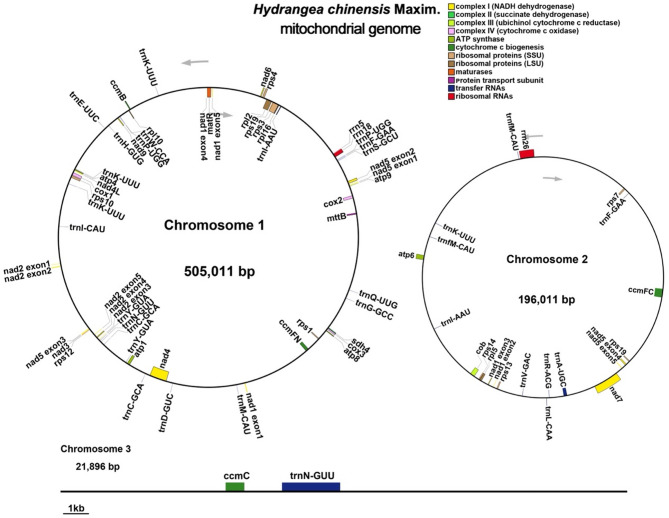
The mitochondrial genome of H. chinensis was meticulously annotated, resulting in the identification of 37 unique protein-coding genes, with the exception of the rps19 gene, which has two copies. This annotation encompasses 24 distinct core mitochondrial genes and 14 non-core genes, as well as 21 tRNA genes (with eight being multi-copy) and three rRNA genes (Fig. 2). Among the core genes, we identified five ATP synthase genes (atp1, atp4, atp6, atp8, and atp9); nine NADH dehydrogenase subunit genes (nad1, nad2, nad3, nad4, nad4L, nad5, nad6, nad7, and nad9); four cytochrome c biogenesis genes (ccmB, ccmC, ccmFC, and ccmFN); three cytochrome c oxidase subunit genes (cox1, cox2 and cox3); a single membrane transporter protein gene (mttB); one maturase gene (matR), and one ubiquinol-cytochrome c reductase gene (cob). The non-core segment includes four large ribosomal subunit genes (rpl2, rpl5, rpl10 and rpl16) and nine small ribosomal subunit genes (rps1, rps3, rps4, rps7, rps10, rps12, rps13, rps14 and rps19), alongside a solitary succinate dehydrogenase gene (sdh4) (Table 2).
Fig. 2.
H. chinensis mitochondrial genome and gene annotation map. The mitochondrial genome is depicted as a circular map illustrating gene organization and transcriptional orientation, with arrows indicating the direction of transcription. Genes are color-coded according to their functional categories: protein-coding genes, tRNA genes, rRNA genes, and others. The map highlights the structural complexity of the mitogenome while providing an overview of its annotated elements
Table 2.
Coding genes of the H. chinensis mitochondrial genome
| Group of genes | Name of genes |
|---|---|
| ATP synthase | atp1, atp4, atp6, atp8, atp9 |
| NADH dehydrogenase | nad1, nad2, nad3, nad4, nad4L, nad5, nad6, nad7, nad9 |
| Cytochrome b | cob |
| Cytochrome c biogenesis | ccmB, ccmC, ccmFC, ccmFN |
| Cytochrome c | oxidase cox1, cox2, cox3 |
| Maturases | matR |
| Protein transport subunit | mttB |
| Ribosomal protein large subunit | rpl2, rpl5, rpl10, rpl16 |
| Ribosomal protein small subunit | rps1, rps3, rps4, rps7, rps10, rps12, rps13, rps14, rps19 (×2) |
| Succinate dehydrogenase | sdh4 |
| Ribosome RNA | rrn5, rrn18, rrn26 |
| Transfer RNA | trnA-UGC, trnC-GCA(×2), trnD-GUC, trnE-UUC, trnF-GAA (×2), trnfM-CAU (×2), trnG-GCC, trnH-GUG, trnI-AAU (×2), trnI-CAU, trnK-UUU (×4), trnL-CAA, trnM-CAU, trnN-GUU (×2), trnP-UGG (×2), trnQ-UUG, trnR-ACG, trnS-GCU, trnV-GAC, trnW-CCA, trnY-GUA (×2) |
The numbers in parentheses represent the copy number of the gene, such as (×2) indicating two copies
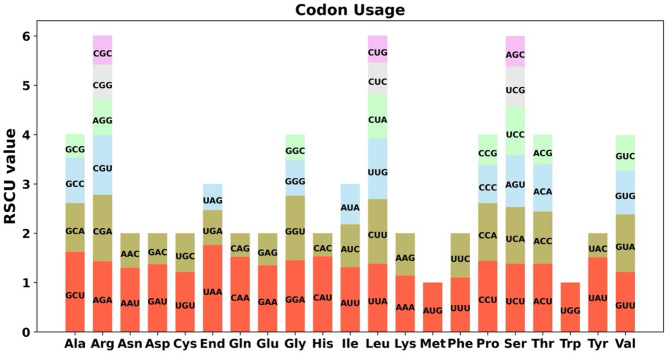
Codon preference refers to the differential frequency with which synonymous codons are employed during the translation process and the evolutionary establishment of a set of preferred codons that align with it, playing a crucial role in gene expression. There is a marked variation in codon usage among different species. Consequently, this study calculated the Relative Synonymous Codon Usage (RSCU) to investigate codon preference in the 38 protein-coding genes (PCGs) of the H. chinensis mitochondrial genome. The usage distribution for each amino acid across various codons is detailed in Table S1. Codons with an RSCU value greater than 1 are considered to be preferentially used by specific amino acids. As depicted in Fig. 3, aside from the start codon AUG and tryptophan (UGG), both of which possess an RSCU value of 1, there is an evident pattern of preferred codon usage within the mitochondrial PCGs. For instance, the stop codon UAA exhibits a strong preference, boasting the highest RSCU value within mitochondrial PCGs at 1.76. Similarly, alanine (Ala) shows a pronounced preference for GCU, with an RSCU value of 1.56. To further explore codon usage patterns and their relationship with closely related species, we conducted a Codon Usage Bias Analysis on the Mitochondrial Genome of H. macrophylla (see Table S2 and Fig S4). The comparison revealed a remarkably high consistency in codon usage preferences between these two species, indicating that codon usage exhibits substantial uniformity within congeneric species.
Fig. 3.
Codon usage bias analysis of the mitochondrial genome of H. chinensis. This figure illustrates the relative synonymous codon usage (RSCU) values for protein-coding genes within the mitochondrial genome of H. chinensis. The codon usage patterns are depicted for 20 standard amino acids as well as termination (stop) codons, denoted as “End.” Elevated RSCU values highlight a preferential selection for specific codons over their synonymous counterparts, shedding light on translational efficiency and potential adaptive biases in the organism’s mitochondrial genome
Repetitive sequences in the mitochondrial genome
Repetitive sequences play a pivotal role not only in maintaining the higher-order structure of genomes but also in driving evolution, inducing variation, and regulating gene expression. Consequently, we conducted an analysis of dispersed repeats, microsatellites, and tandem repeats within the H. chinensis organelle genome (Fig. 4A). Across the three mitochondrial contigs, we identified 162, 41, and 8 SSRs (Simple Sequence Repeats), respectively. Tetrameric SSRs were found to be the most abundant, whereas hexameric SSRs were the least common. In Chromosome 1, there are 20 tandem repeat sequences with a match ratio exceeding 70%, ranging in length from 9 to 39 base pairs. Additionally, there are 335 repeat pairs with lengths greater than or equal to 30 base pairs; among these, palindromic repeats account for 156 pairs and forward repeats for 179 pairs. Chromosome 2 contains nine tandem repeat sequences with a match ratio above 81%, spanning lengths from 11 to 30 base pairs. This chromosome features 69 repeat pairs longer than or equal to 30 base pairs—39 palindromic and 30 forward repeats. Notably, Chromosome 3 lacks any repeat sequence pairs exceeding or equalling a length of 30 base pairs as well as any other scattered repetitive sequences (Fig. 4B). Figure 5, in particular, illustrates the detailed genomic positional distribution of various repeat sequences, such as palindromic repeats and forward repeats. Through further examination of these repeat sequences across different contigs (Figure S5), our analysis reveals that there are no substantial long segment duplications within the mitochondrial genomes of this species, with the longest repeat segment measuring merely 482 base pairs. The abundance of repetitive sequences provides crucial data for selecting molecular markers that could be used to study the genetic diversity of hydrangeas.
Fig. 4.
Bar Chart analysis of repeat sequences. A The x-axis represents mitochondrial molecules, while the y-axis denotes the number of repeat fragments. The legend is as follows: gray indicates mononucleotide SSRs, yellow represents dinucleotide SSRs, blue signifies trinucleotide SSRs, orange denotes tetranucleotide SSRs, purple symbolizes pentanucleotide SSRs, and red stands for hexanucleotide SSRs. B The x-axis again corresponds to mitochondrial molecules, with the y-axis depicting the number of repeat fragments. The legend is defined as: blue for tandem repeats, purple for palindromic repeats, green for forward repeats, red for reverse repeats, and gray for complementary repeats
Fig. 5.
Chord diagram analysis of repeat sequences. Repeat sequence analysis was conducted for three mitochondrial molecules, with Chromosome 1 serving as an exemplar. In the innermost circle, colored lines connect the two sequences of dispersed repeats: purple lines indicate palindromic repeats, while green lines denote forward repeats. The second circle features black segments representing tandem repeat sequences, and the outermost circle contains black segments indicating microsatellite repeat sequences. This pattern continues similarly for Chromosome 2 through Chromosome 3
Prediction of RNA editing sites
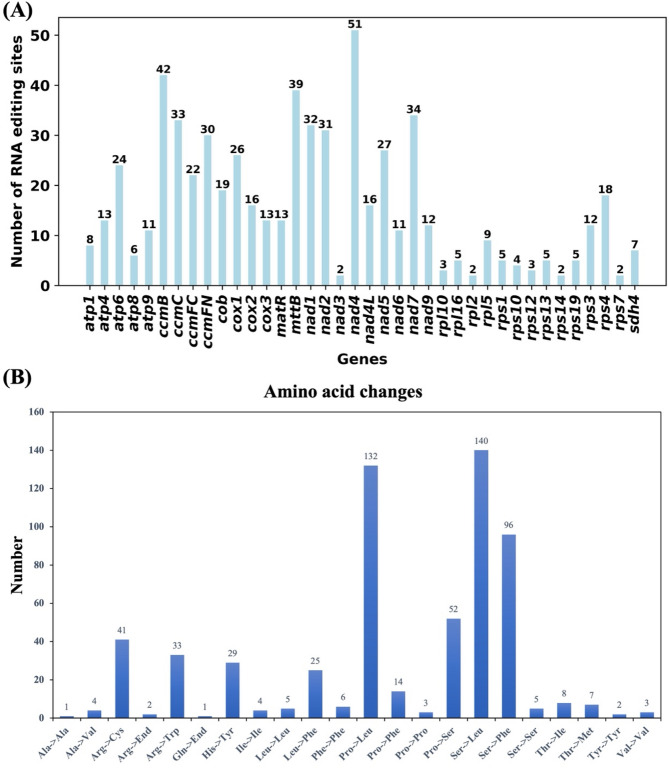
RNA editing is a process characterized by the insertion, deletion, or alteration of nucleotides during DNA transcription, occurring in mitochondria, chloroplasts, and nuclei to form RNA. Utilizing the Deepred-mt tool, we identified RNA editing events within 38 unique protein-coding genes (PCGs) from H. chinensis mitochondria. These genes include atp1, atp4, atp6, atp8, atp9, ccmB, ccmC, ccmFC, ccmFN, cob, cox1, cox2, cox3, matR, mttB, nad1 through nad7 and nad9, rpl10 and rpl16 through rps7 as well as sdh4 (Fig. 6A and Table S3). Ultimately applying a selection threshold greater than 0.9 led to the identification of 613 potential RNA editing sites—all involving C-to-U base conversions. Among the mitochondrial genes analyzed, nad4 exhibited the highest frequency of RNA editing with 51 identified sites. This was followed by the ccmB gene with 42 detected editing sites. Conversely, nad3 and genes such as rpl2, rps14 and rps7 showed minimal editing activity with merely two events each. Notably among these events were 29 synonymous mutations where nucleotide substitutions did not alter the corresponding amino acids. Furthermore, there were two instances exceeding 100 occurrences: Proline transforming into Leucine and Serine transitioning into Leucine (Fig. 6B). These RNA editing events have the potential to alter protein functionality, possibly impacting their roles in mitochondrial respiration and energy production.
Fig. 6.
Overview of RNA editing events. A Distribution of predicted RNA editing sites across various mitochondrial protein-coding genes (PCGs). B Distribution of the number of amino acid changes
Prediction of mitochondrial plastid DNA
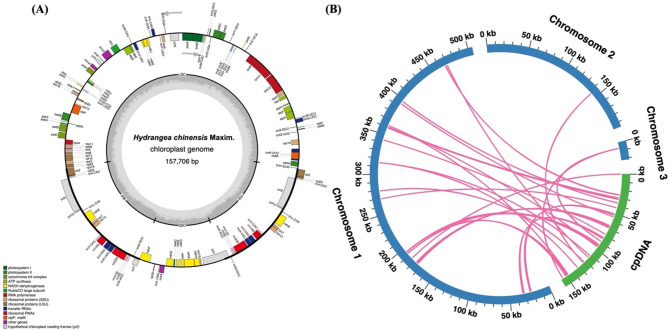
Mitochondrial plastid DNAs (MTPTs) are remnants of chloroplast-origin DNA within mitochondrial genomes, indicating segments originally derived from the chloroplast genome subsequently integrated into the mitochondrial genome. In this study, we employed a methodology akin to that used for mitochondria to assemble the chloroplast genome of H. chinensis, which spans a length of 157,706 base pairs. This assembly revealed predictions for 88 protein-coding genes, 57 tRNA genes, and 8 rRNA genes (Fig. 7A). Through sequence similarity analysis within the hydrangea species, we identified a total of 23 homologous fragments shared between the mitochondrial and chloroplast genomes, with a cumulative length of 11,059 base pairs—comprising 1.53% of the mitochondrial genome’s total length (Fig. 7B and Table S4). Among these fragments, MTPT1 emerged as the longest at 2,942 base pairs. Annotation of these homologous sequences uncovered 18 complete genes across the 23 homologous segments. These include 11 protein-coding genes (ndhJ, ndhK, petL, petN, psbE, psbF, psbJ, psbL, psbM, rpl14 and ycf15) and an array of seven tRNA genes (trnD-GUC, trnH-GUG, trnI-CAU, trnM-CAU trnN-GUU trnP-UGG trnW-CCA). These findings underscore the intricate genomic interactions between the mitochondrial and chloroplast genomes, highlighting the evolutionary significance of gene transfer events in shaping the genomic architecture of hydrangea.
Fig. 7.
Overview of sequence transfer. A Chloroplast genome and gene annotation map. B Circos plot of sequence transfer. In the diagram, blue arcs represent the mitochondrial genome, while green arcs denote the chloroplast genome. The pink connecting lines between arcs correspond to homologous genomic segments
Phylogenetic relationships
To elucidate the evolutionary history of H. chinensis, we constructed a phylogenetic tree using the mitochondrial genomes of 29 species across four orders of angiosperms. Initially, we identified 19 conserved mitochondrial protein-coding genes’ DNA sequences through shared gene identification: atp1, atp4, atp6, atp8, atp9, ccmB, ccmFC, ccmFN, cob, cox2, cox3, matR, nad1, nad3, nad5, nad6, nad7, rpl5 and rps12. Using two Santalales mitochondrial genomes as outgroups allowed for the construction of a phylogenetic tree (Fig. 8A). The phylogenetic analysis demonstrated that H. chinensis belongs to the order Cornales within the family Hydrangeaceae and occupies a phylogenetic position between Caryophyllales and Ericales. This topology based on mitochondrial DNA aligns with the latest classification by APG (Angiosperm Phylogeny Group). The complete mitochondrial genome of H. chinensis represents a valuable asset for developing molecular markers and enhancing our understanding of its evolutionary history and cultivation strategies.
Fig. 8.
Phylogenetic Relationships and Chromosomal Recombination Events in H. chinensis. A Phylogenetic Analysis. A maximum likelihood (ML) phylogenetic tree was constructed based on 19 conserved protein-coding genes (atp1, atp4, atp6, atp8, atp9, ccmB, ccmFC, ccmFN, cob, cox2, cox3, matR, nad1, nad3, nad5, nad6, nad7, rpl5, rps12) from the mitochondrial genomes of 29 plant species. The numbers above the nodes represent support values, including ML bootstrap values and branch lengths. The blue region denotes Ericales, the pink region signifies Cornales, the green region represents Caryophyllales, and the orange region indicates Santalales; the Hydrangea studied is highlighted in red. B Syntenic Blocks Between H. chinensis and Five Closely Related Species. The red arc regions indicate areas where inversions have occurred, while gray regions represent areas of high homology. For clarity in presentation, syntenic blocks shorter than 0.5 kb were not included in the results
Further analysis of collinear regions within organelle genomes from six representative species across different families and orders revealed an abundance of homologous collinear segments. To enhance the clarity of our findings, we excluded collinear blocks shorter than 0.5 kb from the presented results (Fig. 8B). Our investigations detected homologous collinear blocks between hydrangea and its phylogenetically related species, albeit these blocks were relatively short. The findings indicate a lack of consistency in the arrangement of mitochondrial genome collinear blocks among these six species.
Remarkably, synteny between the mitochondrial genomes of hydrangeas and their closely related species is relatively poor, marked by extensive genome rearrangements and substantial structural differences. For example, a mere 214 collinear regions were discerned between H. chinensis and M. jalapa, collectively spanning just 107,839 base pairs (bp). In stark contrast, comparisons involving H. macrophylla and C. nitidissima uncovered as many as 581 collinear regions; however, their total extent reached only 207,594 bp. Among the hydrangea species themselves, the mitochondrial genome synteny between H. chinensis and H. macrophylla is comparatively better—exhibiting 414 shared segments with a total size of 494,614 bp. Nevertheless, even within different species of the Hydrangea genus, significant genomic rearrangement events are evident in their mitochondria, indicating pronounced differences remain. This suggests that these two hydrangea are genetically distinct from each other and possess evolutionary relationships that are more distant than previously anticipated.
Discussion
Structural complexity and recombination-driven diversity in the mitochondrial genome
The mitochondrial genome of H. chinensis exhibits a complex structure, characterized by three contigs, two of which can form circular configurations, while one remains linear. This multi-branched architecture is consistent with the structural plasticity observed in other plant mitochondrial genomes, which often undergo recombination and rearrangement events due to the presence of large repeat sequences and homologous recombination mechanisms [42, 43]. The identification of 335 repeat pairs, including palindromic and forward repeats, highlights the potential for frequent recombination, which may drive genomic rearrangements and contribute to the structural diversity of the mitochondrial genome. These findings align with previous studies on plant mitochondrial genomes, where recombination events have been shown to play a crucial role in genome evolution and adaptation [44]. The presence of numerous simple sequence repeats (SSRs) further underscores the dynamic nature of the mitochondrial genome. SSRs are known to contribute to genome instability and variability, which in turn can influence gene expression and mitochondrial function [45]. The abundance of SSRs in H. chinensis provides a valuable resource for the development of molecular markers, which could be used to study genetic diversity and phylogenetic relationships within the Hydrangea genus and across related species.
RNA editing and its role in mitochondrial function
Our analysis of RNA editing events in the mitochondrial genome of H. chinensis revealed a total of 613 C-to-U editing sites, a phenomenon commonly observed in plant mitochondria [46]. These RNA editing events are known to play a critical role in post-transcriptional regulation by altering codons, which can lead to changes in amino acid sequences, the creation of new start or stop codons, and ultimately the modification of protein function [46]. In H. chinensis, the nad4 gene exhibited the highest number of editing sites, suggesting that this gene may undergo extensive post-transcriptional modifications, potentially influencing its role in mitochondrial respiration and energy production. The identification of 29 synonymous mutations among the RNA editing events indicates that not all editing events result in functional changes at the protein level. However, the non-synonymous editing events observed in key mitochondrial genes, such as cox1, atp6, and ccmB, suggest that RNA editing may be essential for the proper functioning of the electron transport chain and other mitochondrial processes. These findings are consistent with previous studies on RNA editing in plant mitochondria, where editing has been shown to be crucial for maintaining mitochondrial integrity and function [47].
Evidence of chloroplast-to-mitochondrial DNA transfer
The detection of 23 mitochondrial plastid DNA (MTPT) fragments in the H. chinensis mitochondrial genome provides evidence of gene transfer from the chloroplast genome, a process that is well-documented in plant organelles [48]. These MTPTs, which account for 1.53% of the mitochondrial genome, include both protein-coding genes and tRNA genes, suggesting that chloroplast-derived sequences may have been co-opted by the mitochondrial genome over evolutionary time. The presence of MTPTs highlights the dynamic nature of plant mitochondrial genomes, which are known to incorporate foreign DNA from various sources, including chloroplasts and even the nuclear genome [49]. The functional significance of these MTPTs remains unclear, but their integration into the mitochondrial genome may contribute to genomic rearrangements and the generation of novel genetic combinations. Additionally, the presence of chloroplast-derived tRNA genes in the mitochondrial genome may play a role in optimizing mitochondrial translation processes, although further research is needed to elucidate the exact functions of these transferred sequences.
Phylogenetic insights and evolutionary dynamics of H. chinensis
Our phylogenetic analysis, based on 19 conserved mitochondrial protein-coding genes, positioned H. chinensis within the order Cornales, in agreement with the latest APG (Angiosperm Phylogeny Group) classification [50]. This confirms the phylogenetic placement of H. chinensis and provides further evidence of the evolutionary relationships between hydrangea and other members of the Cornales order. The close phylogenetic relationship between H. chinensis and species from the families Caryophyllaceae and Ericaceae, as revealed by collinearity analysis, suggests that these species may share common evolutionary mechanisms, particularly in terms of mitochondrial genome rearrangements and RNA editing events. Interestingly, our comparative analysis of collinear regions across six representative species revealed significant genomic rearrangements between H. chinensis and its phylogenetically related species, particularly in the mitochondrial genome. These findings suggest that the mitochondrial genomes of hydrangea species have undergone extensive structural divergence, possibly driven by recombination events, gene transfer, and selection pressures. The observed divergence between mitochondrial genomes of different hydrangea further emphasizes the genetic complexity and evolutionary plasticity within this genus.
Conclusions
This study presents the first comprehensive assembly and characterization of the H. chinensis mitochondrial genome, providing valuable insights into its structural complexity and evolutionary dynamics. Our findings reveal extensive genomic recombination and the integration of chloroplast-derived segments, underscoring the dynamic nature of the mitogenome in this species. The identification of numerous RNA editing sites, including those affecting start and stop codons, highlights the potential for post-transcriptional regulation of mitochondrial gene expression in H. chinensis. Collectively, these results not only enhance our understanding of mitochondrial genome organization and evolution within the Hydrangea genus but also establish a foundational resource for future comparative genomics and molecular breeding efforts in ornamental plants.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors sincerely thank the experimental personnel of Wuhan Benagen Technology Co., Ltd (https://www.benagen.com/) for their participation in this project.
Author contributions
Kang Ye undertook the assembly of the mitogenome, conducted data analysis, and composed the manuscript. All authors have thoroughly read and given their approval for the final version of the manuscript. Jun Qin meticulously conceived the project and provided a comprehensive review of the manuscript. Yonghong Hu meticulously conceived the project.
Funding
This work was supported by Special Fund for Scientific Research of National Botanical Gardens to benefit sustainable development (XMD4-12-001) and Special Fund for Scientific Research of Shanghai Landscaping & City Appearance Administrative Bureau (G242424).
Data availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material. The mitogenome sequence supporting the conclusions of this article is available in GenBank (https://www.ncbi.nlm.nih.gov/) under accession number: PQ593888, PQ593889, PQ593890. The chloroplast genome sequence information has been uploaded to NCBI (accession number: PQ593891).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Permission for material collection
We are licensed to collect the plant of Hydrangea chinensis from Shuangxi Town in Pingnan County, Fujian Province, China (E119°03’19’’, N27°03’28”, elevation 924 m). The collection was conducted by the Shanghai Chenshan Botanical Garden and complies with relevant institutional, national, and international guidelines and legislation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nashima K, Shirasawa K, Ghelfi A, Hirakawa H, Isobe S, Suyama T, Wada T, Kurokura T, Uemachi T, Azuma M, Akutsu M, Kodama M, Nakazawa Y, Namai K. Genome sequence of hydrangea macrophylla and its application in analysis of the double flower phenotype. DNA Res. 2020;28: dsaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinehart TA, Wadl PA, Staton ME. An update on hydrangea macrophylla breeding targets and genomics. Acta Hortic. 2018;1191:217–24. [Google Scholar]
- 3.Khalil AT, Chang FR, Lee YH, Chen CY, Liaw CC, Ramesh P, Yuan SSF, Wu YC. Chemical constituents from the Hydrangea chinensis. Arch Pharm Res. 2003;26:15–20. [DOI] [PubMed] [Google Scholar]
- 4.Yang TL, Kao CL, Kuo CE, Yeh HC, Li HT, Li WJ, Chen CY. Secondary metabolites of hydrangea chinensis. Chem Nat Compd. 2021;57:548–50. [Google Scholar]
- 5.Annesley SJ, Fisher PR. Mitochondria in health and disease. Cells. 2019;8:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole LW, Guo W, Mower JP, Palmer JD. High and variable rates of repeat-mediated mitochondrial genome rearrangement in a genus of plants. Mol Biol Evol. 2018;35:2773–85. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Chen H, Ni Y, Li J, Cai Y, Ma B, Yu J, Wang J, Liu C. De Novo hybrid assembly of the salvia miltiorrhiza mitochondrial genome provides the first evidence of the Multi-Chromosomal mitochondrial DNA structure of salvia species. Int J Mol Sci. 2022;23:14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lower SE, Dion-Côté AM, Clark AG, Barbash DA. Special issue: repetitive DNA sequences. Genes. 2019;10:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller H, Ogereau D, Da Lage J-L, Capdevielle C, Pollet N, Fortuna T, Jeannette R, Kaiser L, Gilbert C. Draft nuclear genome and complete mitogenome of the Mediterranean corn borer, Sesamia nonagrioides, a major pest of maize. G3 Genes|Genomes|Genetics. 2021;11: jkab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipović I, Hereward JP, Rašić G, Devine GJ, Furlong MJ, Etebari K. The complete mitochondrial genome sequence of Oryctes rhinoceros (Coleoptera: Scarabaeidae) based on long-read nanopore sequencing. PeerJ. 2021;9: e10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han F, Qu Y, Chen Y, Xu L, Bi C. Assembly and comparative analysis of the complete mitochondrial genome of Salix wilsonii using PacBio HiFi sequencing. Front Plant Sci. 2022;13:1031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Štorchová H, Stone JD, Sloan DB, Abeyawardana OAJ, Müller K, Walterová J, Pažoutová M. Homologous recombination changes the context of Cytochrome b transcription in the mitochondrial genome of Silene vulgaris KRA. BMC Genomics. 2018;19:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu Y, Zhou P, Tong C, Bi C, Xu L. Assembly and analysis of the Populus deltoides mitochondrial genome: the first report of a multicircular mitochondrial conformation for the genus Populus. J Forestry Res. 2022;34:717–33. [Google Scholar]
- 14.Small ID, Schallenberg‐Rüdinger M, Takenaka M, Mireau H, Ostersetzer‐Biran O. Plant organellar RNA editing: what 30 years of research has revealed. Plant J. 2019;101:1040–56. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Zhang Q, Yin P. RNA editing machinery in plant organelles. Sci China Life Sci. 2017;61:162–9. [DOI] [PubMed] [Google Scholar]
- 16.Gandini CL, Sanchez-Puerta MV. Foreign plastid sequences in plant mitochondria are frequently acquired via mitochondrion-to-mitochondrion horizontal transfer. Sci Rep. 2017;7:43402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sloan DB, Wu Z. History of plastid DNA insertions reveals weak deletion and AT mutation biases in angiosperm mitochondrial genomes. Genome Biol Evol. 2014;6:3210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang B, Li J, Zhao Q, Liang Y, Yu J. Assembly of the complete mitochondrial genome of Chinese plum (Prunus salicina): characterization of genome recombination and RNA editing sites. Genes. 2021;12:1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porebski S, Bailey LG, Baum BR. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep. 1997;15:8–15. [Google Scholar]
- 20.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37:540–6. [DOI] [PubMed] [Google Scholar]
- 21.Wick RR, Schultz MB, Zobel J, Holt KE. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 2015;31(20):3350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM; 2013. ArXiv Preprint. arXiv:13033997.
- 23.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. Geseq– versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017;45:W6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Ye W, Zhang Y, Xu Y. High speed BLASTN: an accelerated megablast search tool. Nucleic Acids Res. 2015;43:7762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis SE, Searle SMJ, Harris N, Gibson M, Iyer V, Richter J, Wiel C, Bayraktaroglu L, Birney E, Crosby MA, Kaminker JS, Matthews BB, Prochnik SE, Smith CD, Tupy JL, Rubin GM, Misra S, Mungall CJ, Clamp ME. Apollo: a sequence annotation editor. Genome Biol. 2002;3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT. Phylosuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 2019;20:348–55. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beier S, Thiel T, Münch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction. Bioinformatics. 2017;33:2583–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtz S. REputer: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;29:4633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Meltzer P, Davis S. RCircos: an R package for circos 2D track plots. BMC Bioinformatics. 2013;14:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin J-J, Yu W-B, Yang J-B, Song Y, dePamphilis CW, Yi T-S, Li D-Z. Getorganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 2019;47:W65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Ni Y, Li J, Zhang X, Yang H, Chen H, Liu C. Cpgview: a package for visualizing detailed Chloroplast genome structures. Mol Ecol Resour. 2023;23:694–704. [DOI] [PubMed] [Google Scholar]
- 37.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Letunic I, Bork P. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47:W256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edera AA, Small I, Milone DH, Sanchez-Puerta MV. Deepred-Mt: deep representation learning for predicting C-to-U RNA editing in plant mitochondria. Comput Biol Med. 2021;136: 104682. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Tang H, DeBarry JD, Tan X, Li J, Wang X, Lee T, Jin H, Marler B, Guo H, Kissinger JC, Paterson AH. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40: e49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arrieta-Montiel MP, Mackenzie SA. Plant mitochondrial genomes and recombination. Plant Mitochond. 2011;2010:65–82. [Google Scholar]
- 43.Gualberto JM, Mileshina D, Wallet C, Niazi AK, Weber-Lotfi F, Dietrich A. The plant mitochondrial genome: dynamics and maintenance. Biochimie. 2014;100:107–20. [DOI] [PubMed] [Google Scholar]
- 44.Martin W. Gene transfer from organelles to the nucleus: frequent and in big chunks. Proc Natl Acad Sci U S A. 2003;100:8612–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith DR, Keeling PJ. Mitochondrial and plastid genome architecture: reoccurring themes, but significant differences at the extremes. Proc Natl Acad Sci U S A. 2015;112:10177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonnard G, Gualberto JM, Lamattina L, Grienenberger JM, Brennlcke A. RNA editing in plant mitochondria. CRC Crit Rev Plant Sci. 1992;10:503–24. [Google Scholar]
- 47.Gualberto JM, Newton KJ. Plant mitochondrial genomes: dynamics and mechanisms of mutation. Annu Rev Plant Biol. 2017;68:225–52. [DOI] [PubMed] [Google Scholar]
- 48.Notsu Y, Masood S, Nishikawa T, Kubo N, Akiduki G, Nakazono M, Hirai A, Kadowaki K. The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: frequent DNA sequence acquisition and loss during the evolution of flowering plants. Mol Genet Genomics. 2002;268:434–45. [DOI] [PubMed] [Google Scholar]
- 49.Kubo T, Mikami T. Organization and variation of angiosperm mitochondrial genome. Physiol Plant. 2006;129:6–13. [Google Scholar]
- 50.Chase MW, Christenhusz MJM, Fay MF, Byng JW, Judd WS, Soltis DE, Mabberley DJ, Sennikov AN, Soltis PS, Stevens PF. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc. 2016;181:1–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material. The mitogenome sequence supporting the conclusions of this article is available in GenBank (https://www.ncbi.nlm.nih.gov/) under accession number: PQ593888, PQ593889, PQ593890. The chloroplast genome sequence information has been uploaded to NCBI (accession number: PQ593891).