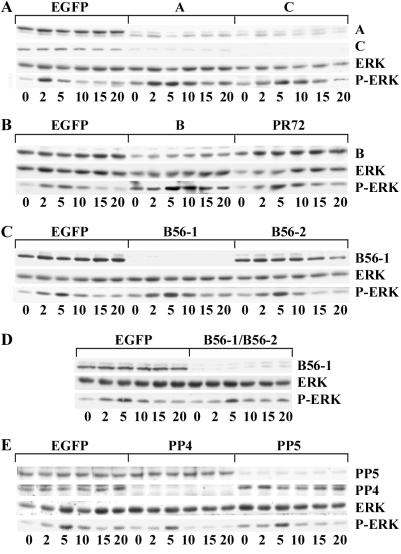
Figure 3.
PP2A negatively regulates insulin-dependent ERK activation. Drosophila S2 cells were incubated in the presence of dsRNA targeted to EGFP or to the specific phosphatase subunits indicated across the tops of A–E. After 72 h, cells were treated with 10 μg/ml insulin for the minutes indicated at the bottoms of A–E and lysed in RIPA buffer containing 0.2 mM Na2VO4 and 10 mM NaF2. Lysates were clarified by centrifugation, and equal amounts of protein (50 μg) were resolved by SDS/PAGE. Proteins were transferred to nitrocellulose membranes and Western blotted with antibodies against the PP2A-A, -C, -R2/B, -R5/B56–1, PP4, or PP5 as indicated at the right of each row. MAP kinase activation was detected by blotting with antibodies specific for the phosphorylated forms of ERK-1 and -2 (P-ERK). The amount of MAP kinase in each sample was determined by blotting with an antibody that recognizes both the phosphorylated and nonphosphorylated forms of ERK-1 and ERK-2. Results shown are representative of three to five experiments for each dsRNA treatment.