Abstract
Background
Posterior reversible encephalopathy syndrome is a rare disorder encompassing multiple neurological symptoms usually corroborated by specific neuro magnetic resonance imaging features. Posterior reversible encephalopathy syndrome may be triggered by multiple clinical situations such as blood pressure fluctuations, ischemic stroke, inflammatory and autoimmune disorders, renal failure, pre-eclampsia and eclampsia, hematopoietic stem cell transplantation, cytotoxic drugs, calcineurin inhibitors (cyclosporine A), and other immunosuppressants, as well as a wide range of surgical procedures (mainly cranial and solid organ transplantation). Although rare after cardiac transplantation, posterior reversible encephalopathy syndrome remains a major adverse event among feared complications promoted by use of immunosuppressive drugs. Clinical symptomatology, imaging features, and evolution of posterior reversible encephalopathy syndrome as well therapeutic strategy and identification of contributing factors will be discussed on the basis of our experience and data from literature review.
Case presentation
We report the case of a 59-year-old white male patient who was diagnosed with posterior reversible encephalopathy syndrome 3 months after cardiac transplantation. Neurologic complications gradually worsened within weeks after transplantation from an immediate postoperative paraparesis to seizures and coma requiring specific management in the intensive care unit. Initial brain computed tomography and magnetic resonance imaging were not contributive. Ultimately, magnetic resonance imaging characteristics of posterior reversible encephalopathy syndrome gradually appeared 10 weeks after transplantation and were concomitant with epileptic seizures, coma, and occurrence of Shiga toxin-producing Escherichia coli-hemolytic–uremic syndrome in a context of blood pressure variations and administration of cyclosporine A.
Conclusion
This case highlighted the necessity for clinicians to be familiar with posterior reversible encephalopathy syndrome to prevent misdiagnosis and optimize neurological outcomes. In addition, it emphasized the underlying non-dose-dependent neurotoxicity of cyclosporine A.
Keywords: Posterior reversible encephalopathy syndrome, Hemolytic uremic syndrome, Shiga toxin-producing Escherichia coli, Calcineurin inhibitors, Cyclosporine A, Tacrolimus, Solid organ transplantation, Status epilepticus, Cerebral MRI
Introduction
Posterior reversible encephalopathy syndrome (PRES) also named reversible posterior leukoencephalopathy syndrome (RPLS) is an infrequent neurologic complication that occurs in response to multiple factors. In 1996, Hinchey and colleagues [1] first coined the term posterior reversible encephalopathy syndrome to describe this complication characterized by a large spectrum of clinical features with distinct neuroradiological findings in the parieto-occipital area. PRES is also referred to other syndromic appellations such as reversible posterior cerebral edema syndrome, hyperperfusion encephalopathy, brain capillary leak syndrome, which involve a mechanism of vasogenic edema with brain perfusion disorders [2–6].
PRES usually presents with headache, blurring of vision, cortical blindness, confusion, altered mental confusion, and seizures [7]. However, PRES is mainly a radiological diagnosis, as neuroimaging usually displays characteristic parieto-occipital white matter edema and hyperintensities [8]. Most of the time, it is reversible in 89% of cases [9]; nevertheless, PRES may lead to irreversible cerebral lesions with definitive sequelae in the long term, such as chronic epilepsy or other pejorative adverse events, if misdiagnosed or treatment is delayed. This latter risk is dramatically increased when unusual cerebral matter locations rather than posterior or white matter are identified, thus delaying diagnosis and therapeutic management [3, 10, 11].
PRES has been causally related to a plethora of clinical situations and factors such as renal failure, hypertension, blood pressure variations, autoimmune and inflammatory disorders, hemolytic–uremic syndrome (HUS), cytotoxic drugs, immunosuppressive agents, neurosurgery, stem cell and bone marrow transplantation, and solid organ transplantation (kidney and heart).
PRES in the setting of cardiac transplantation is an exceptional complication, and therefore, guidelines for diagnosis and management still need to be addressed, as etiological mechanisms involving underlying blood pressure fluctuations, neurotoxicity of drugs and immunosuppressants, and infectious processes remain unclear. We present the case of a 59-year-old white male patient diagnosed with PRES 3 months after heart transplantation. Prodromal symptoms, imaging characteristics, and evolution of the PRES, as well as etiological hypotheses and management are discussed in relation to the review of literature.
Case presentation
A 59-year-old white male patient was diagnosed with PRES 3 months after bicaval heart transplantation for end-stage ischemic cardiomyopathy related to acute thrombosis of the left main trunk. While attempts of coronary recanalization and stenting failed, hemodynamic status rapidly worsened despite intra-aortic balloon pump (IABP) and inotropic support. Hence, a decision was made to transfer the patient to our department. Because of a short-term dismal prognosis, following a multidisciplinary discussion, the patient was bridged to heart transplant with venoarterial extracorporeal membrane oxygenation (VA-ECMO) and a few days later underwent a bicaval heart transplantation. At induction of anesthesia, the patient experienced severe hypotension necessitating hemodynamic support with vasopressors and inotropes. In the immediate postoperative period, the patient presented with a paraparesis with acute renal failure and vasoplegia characterized by persistent low systemic vascular resistance despite a normal cardiac index, resulting in profound and uncontrolled vasodilation for 5 days despite high doses of noradrenaline.
Both cerebral computed tomography (CT) scan and magnetic resonance imaging (MRI) revealed neither stroke nor hemorrhage, with no abnormality of the spinal cord. Paraparesis showed progressive recovery approximately 2 weeks after onset, allowing the patient to gait with mobility assistive devices such as crutches and walker. At 35 days postoperatively, when gait ability sufficiently improved, the patient was discharged to a rehabilitation center to follow neurological and cardiac rehabilitation programs to improve his physical fitness and be autonomous. His immunosuppressive treatment consisted of cyclosporine A (Neoral®), prednisolone (Solupred®), and mycophenolate (Cellcept®), and he went home in good condition.
Subsequently, 1 week later, the patient presented to our department with a few day history of nausea, vomiting, and diarrhea with neither fever nor infectious or inflammatory biologic syndromes.
Abdominal CT scan revealed a circumferential wall thickening of the right and transverse colon consistent with banal segmental colitis. Pending results of coprocultures, the patient was discharged home in good condition with symptomatic treatment.
Then, 3 days later, the patient’s condition rapidly deteriorated owing to persisting nausea and vomiting, and diarrhea significantly worsened; therefore, he was urgently transferred to our institution. Gastrointestinal symptoms were found to be accompanied by mental confusion, dysarthria, motor agitation, deglutition disorders, and left-sided facial paralysis. In addition, results of previous coprocultures identified Shiga toxin-producing Escherichia coli (STEC).
Two consecutive cerebral MRIs showed brain periventricular diffuse white matter with increased signal intensity on the T2/fluid-attenuated inversion recovery (FLAIR) sequences and micro bleeds predominately in the left and right temporal areas (Fig. 1A–C). These features were suggestive of a degenerative microvascular leukoencephalopathy; nevertheless, they were unlikely to be consistent with the age of the patient.
Fig. 1.
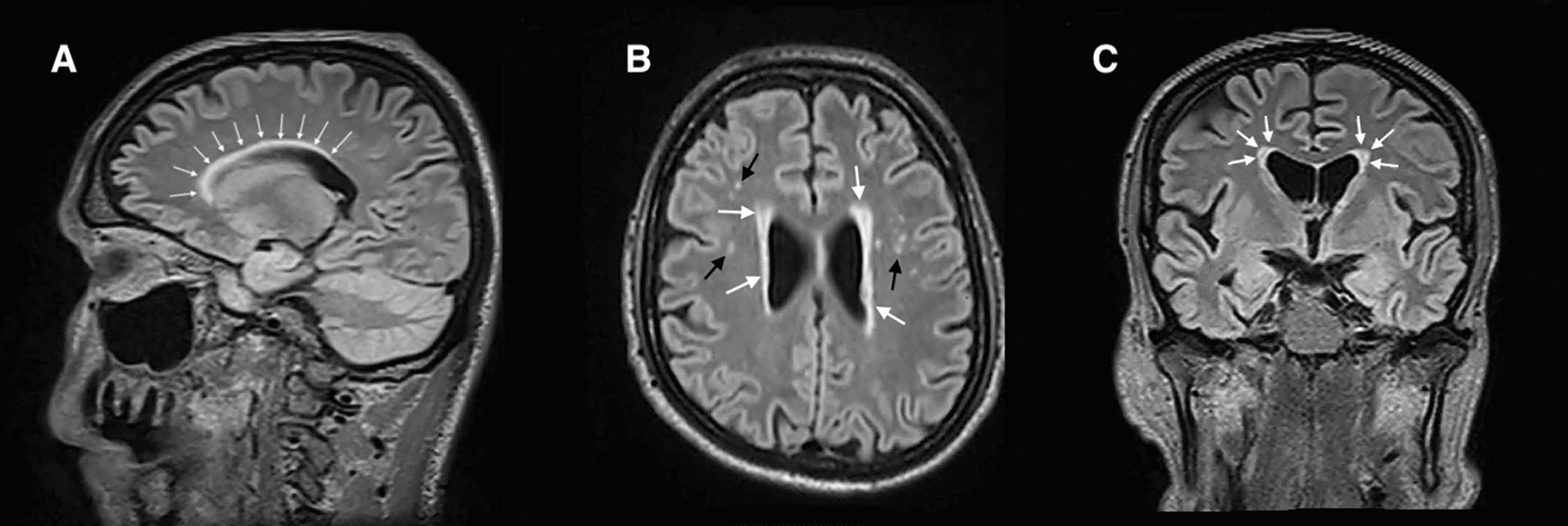
A Sagittal view of brain magnetic resonance imaging in T2/fluid-attenuated inversion recovery sequences showing periventricular increased signal intensity (white arrows). B Axial view of brain magnetic resonance imaging in T2/fluid-attenuated inversion recovery sequences showing periventricular increased signal intensity (white arrows) and micro bleeds in the left and right temporal areas (black arrows). C Frontal view of brain magnetic resonance imaging in T2/fluid-attenuated inversion recovery sequences showing periventricular increased signal intensity (white arrows)
In addition, lumbar punctures (n = 2) showed normal cerebrospinal fluid contents with neither auto- nor anti-onconeural protein antibodies found.
While blood samples did not find any biological inflammatory syndrome (C-reactive protein, < 4 mg/L; leukocyte count, 7200/mm3), hemolytic–uremic syndrome (HUS) was rapidly diagnosed with a triad of thrombocytopenia, hemolytic anemia (schistocytes, 1–2%; haptoglobin, 0.17 g/L), and acute renal failure (serum creatinine, 170 μM/L; proteinuria, 80 mg/L).
Given the associated gastrointestinal prodromal symptoms and enterocolitis associated with STEC, HUS was considered typical HUS (t-HUS) as opposed to atypical HUS (a-HUS) whose clinical presentation, incidence, and etiologies differ.
Therefore, diagnosis of HUS related to STEC, one of two forms of thrombotic microangiopathy (TMA), was suspected, and treatment with eculizumab and azithromycin was started with therapeutic apheresis.
Cyclosporine A, as a drug-etiology-induced TMA, was not initially suspected since residual plasma cyclosporine A levels were 100 μg/L (current recommended values: 150–250 μg/L).
While the clinical situation was improving moderately, 2 weeks later, the patient suddenly developed worsening altered consciousness and coma, requiring endotracheal intubation. No abnormal general movements were observed, and electroencephalograms (EEGs) did not reveal any features of epilepsy and were not contributory in detecting subclinical seizures as a cause of the coma.
Cerebral MRI was comparable to the previous ones, nevertheless with a symmetric hyperintensity of the pulvinar nucleus in T2/FLAIR sequences (Fig. 2A, B), which are assumed to be unusual locations for PRES.
Fig. 2.
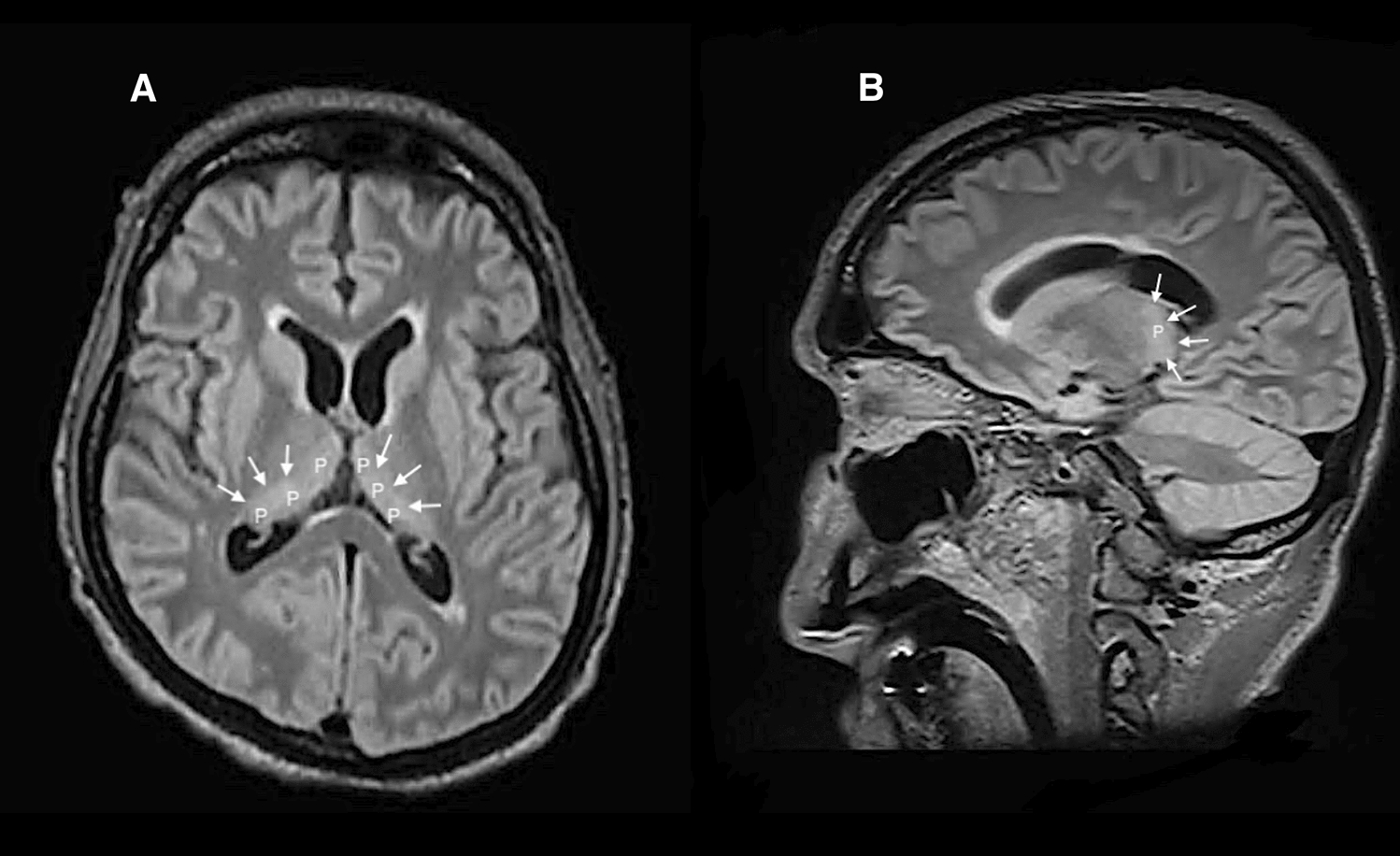
A Axial view of brain magnetic resonance imaging in T2/fluid-attenuated inversion recovery sequences displaying hyperintensity of the pulvinar (P) nuclei (white arrows). B Saggital view of brain magnetic resonance imaging in T2/fluid-attenuated inversion recovery sequences displaying hyperintensity of the pulvinar (P) nuclei (white arrows)
Subsequently, 2 days later, despite sedation, the patient presented with myoclonic tremors of the left arm with seizures, requiring use of lacosamide and levetiracetam. EEG showed features of focal status epilepticus of the right occipital lobe. Necessity to sedate the patient and control seizures produced blood pressure fluctuations requiring continuous hemodynamic support with vasopressors.
Despite pharmacologic treatments, myoclonic tremors persisted, and diagnosis of generalized convulsive status epilepticus was confirmed by EEG, requiring the optimization of sedation by increasing doses of propofol, midazolam, and thiopental, respectively.
Following efficient sedation and despite no myoclonic tremors, daily EEGs showed persistent features of status epilepticus in the left occipital lobe.
After multidisciplinary discussions, a decision was made to transfer the patient to the neuro-intensive care unit (NICU) 3 months after surgery.
In the NICU, while the patient was clinically sedated, three consecutive EEGs showed a progressive onset of interictal epileptiform discharges characterized by propagation of periodical spikes and sharp waves in the right occipital lobe. These EEG features correlated with results of diffusion MRI of the brain showing signal hyperintensity on T2/FLAIR in the white subcortical matter within the right occipital region compatible with PRES (Fig. 3A–E), concomitant with a symmetric persistent hyperintensity of the pulvinar nuclei and periventricular areas.
Fig. 3.
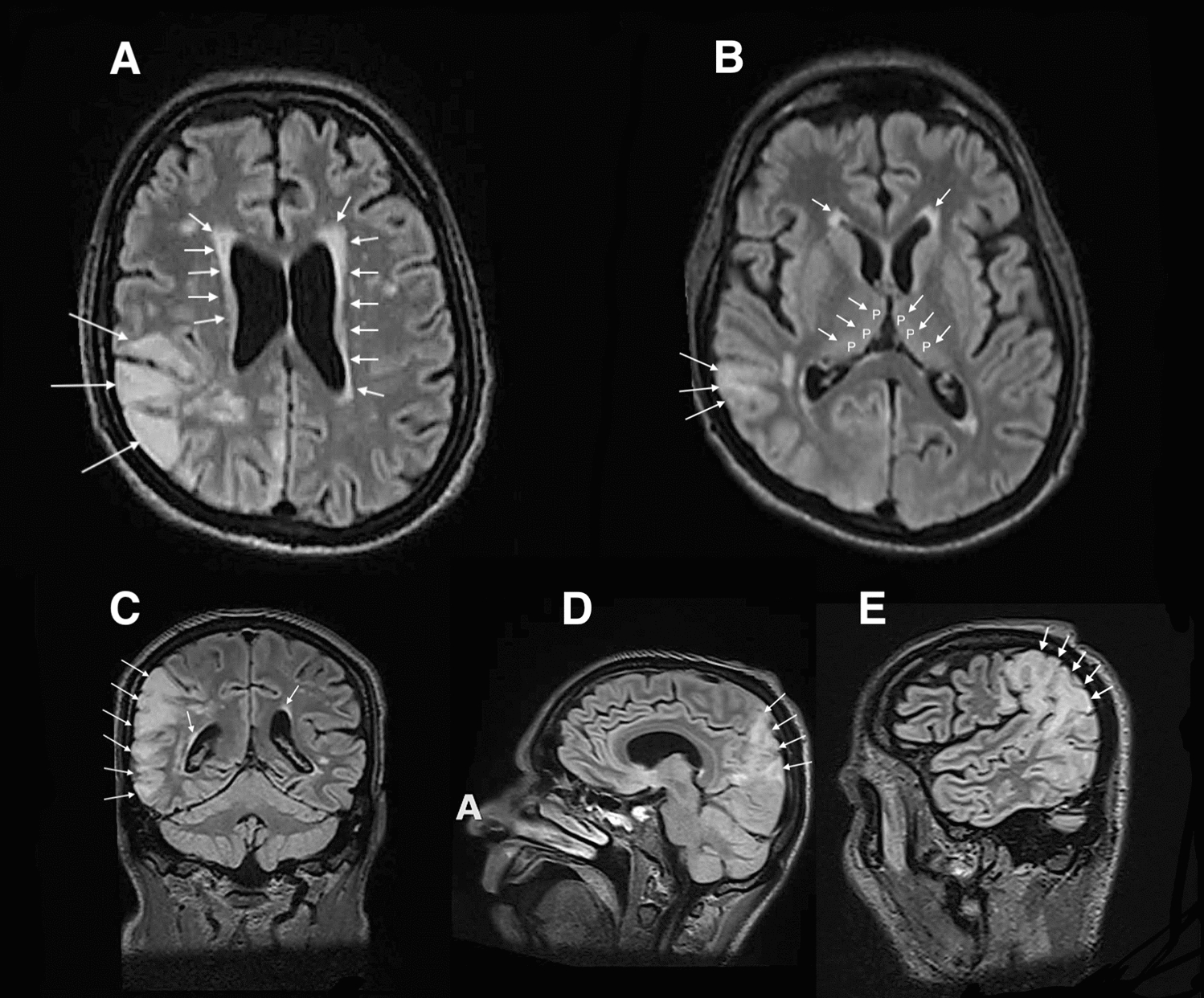
A–E Magnetic resonance imaging of the brain in the T2/fluid-attenuated inversion recovery sequences displaying hyperintensity signals (white arrows) in the white subcortical matter within bilateral occipital region apposite to posterior reversible encephalopathy syndrome and periventricular increased signal intensity (A, B, D). B Persistent hyperintensity of the pulvinar (P) nucleus (white arrows)
Despite low residual plasma levels of cyclosporine A, after discussion and review of literature, a decision was made to interrupt immunosuppression with cyclosporine since it is known to be a drug-etiology-induced TMA and therefore potentially involved in HUS even if previous identification of STEC was probably the main etiology of HUS. Accordingly, cyclosporine A was switched to belatacept.
Within the following days, a steady improvement in clinical status, biological results, EEG monitoring, and MRI features was observed allowing to progressively reduce sedation and ultimately extubate the patient 15 days after being intubated without recurrence of both seizures and major neurological sequelae.
Albeit encouraging recovery, an MRI of the brain, performed 2 weeks and 1 month after extubation, respectively, showed persistent imaging abnormalities that tend to decrease slowly (Fig. 4A–D).
Fig. 4.

A–D Specific imaging features of posterior reversible encephalopathy syndrome characterized by distinctive right parieto-occipital hyperintensity signals and increased periventricular signal (A–C) (white arrows)
Following initial rehabilitation addressing improvement of balance, the patient recovered autonomy and was discharged home.
At 18 months of follow-up, the patient reports minor balance dysfunction, and cerebral MRI shows a significant regression of signal hyperintensity within both the right parieto-occipital area on T2/FLAIR (Fig. 5A to be compared with Fig. 4a) and pulvinar nucleus (Fig. 5B to be compared with Fig. 2B). However, signal hyperintensity is significantly increased within the periventricular white matter, which may be consistent with a mechanism of neurotoxicity.
Fig. 5.
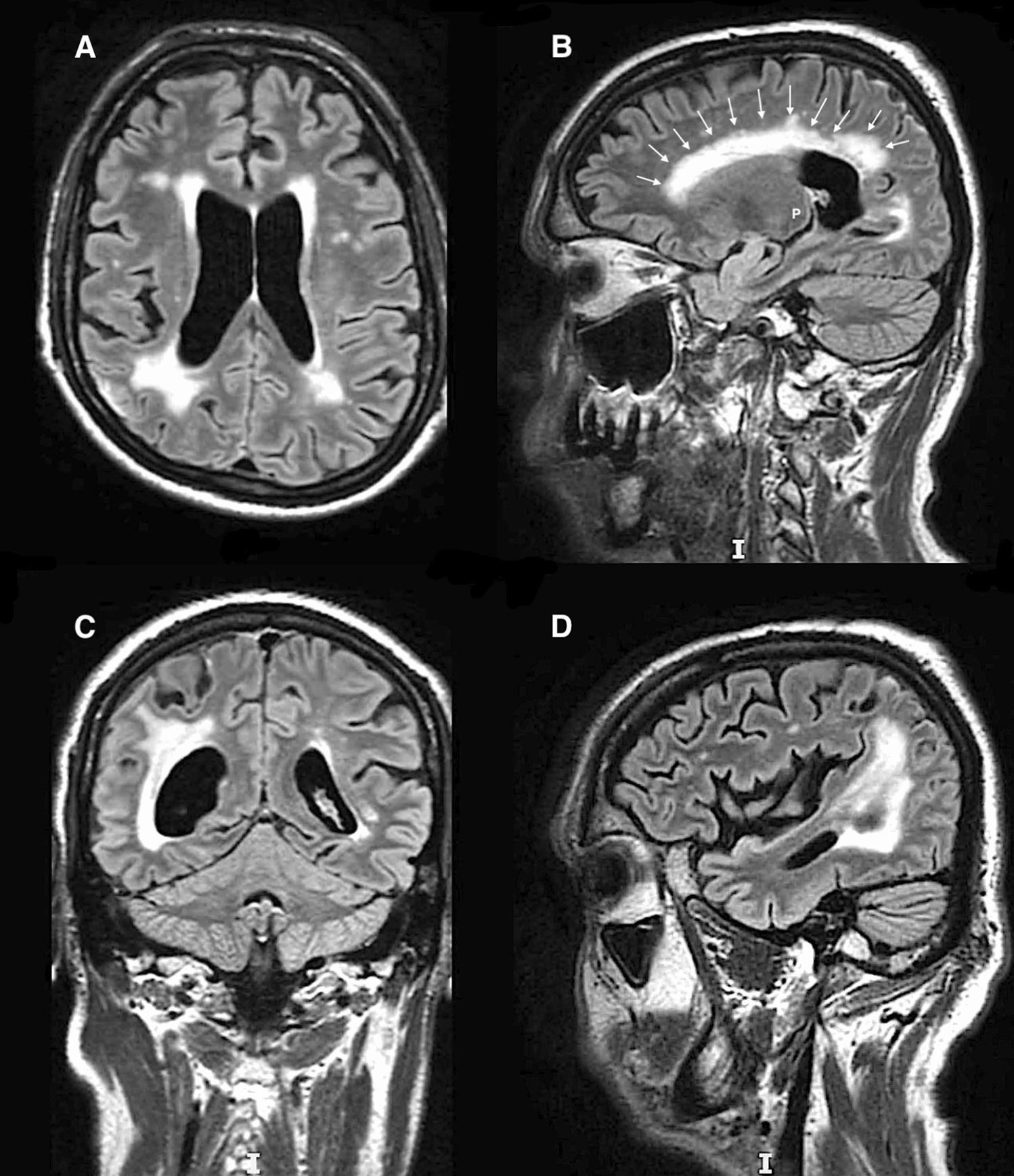
A–D Cerebral magnetic resonance imaging in T2/fluid attenuated inversion recovery sequences showing regression of signal hyperintensity within both the right parieto-occipital area (to be compared with Fig. 4A) and pulvinar nucleus (P) (to be compared with B), while signal hyperintensity is significantly increased within the periventricular white matter (white arrows)
Discussion
PRES is a neurological disorder characterized by the association of neurological symptoms with specific neuroimaging features. Identification of PRES as a specific physiopathological entity is quite recent since it was first described by Hinchey in 1996 [1].
It is considered a major complication caused by a large spectrum of etiologies. These commonly result from promoting factors among which uncontrolled hypertension as well as blood pressure fluctuations are regarded as the main culprits since they are found in about 75% of patients [10]. Most patients have a markedly hypertensive triggering profile; nevertheless, some present with a moderately increased or even normal blood pressure.
Physiopathogenic hypotheses regarding PRES are numerous and unclear, with PRES being likely to result from hemodynamic instability with endothelial dysfunction. Multiple contributory factors and clinical situations, including drugs, refer to these mechanisms and therefore might influence occurrence of PRES.
Table 1 summarizes the most common identified factors and situations causally related to PRES reported in literature. Balcerac et al. in an analysis of 3278 cases of PRES, using VigiBase, the World Health Organization pharmacovigilance database [12], identified 73 molecules statistically associated with PRES. This recent study was a major advancement in identifying suspected drugs associated with PRES and therefore is a precious decision-support tool to help clinicians to decide which drug to discontinue.
Table 1.
Most common identified factors and situations causally related to PRES reported in literature
| • Hypertension |
| • Acute blood pressure fluctuations |
| • Pre-eclampsia and eclampsia |
| • Ischemic stroke |
| • Acute and chronic renal failure |
| • Inflammatory and autoimmune diseases |
| • Hypercalcemia and hypomagnesemia |
| • Chemotherapy and cytotoxic drugs (paclitaxel, dihydrofolate reductase inhibitor |
| • Calcineurin inhibitor (cyclosporine A) |
| • Immunosuppressants (tacrolimus) |
| • Steroids |
| • HUS (hemolytic and uremic syndrome), HUSEC (HUS + STEC) |
| • Porphyria |
| • Thrombotic thrombocytopenic purpura |
| • Iodine contrast exposure (cerebral and coronary angiography) |
| • Surgical procedures (cranial) |
| • Transplantation |
| • Bone marrow |
| • Stem cells |
| • Solid organ (heart, kidney) |
Considering cases of PRES occurring after surgery, no direct relationship between the surgical procedure itself and the onset of PRES was clearly identified [13] as it was not possible to assess whether PRES resulted from surgery-related stress or a mismanagement of general anesthesia during the procedure [14]. Our case illustrates this key point since we could not ascertain the postinduction persistent hypotension to be the primary triggering factor of PRES; other factors were obviously involved such as HUS and cyclosporine A. Nevertheless, particular attention needs to be paid to PRES occurring after surgery, since association of PRES and surgery is known to refer to different situations.
The first one is PRES following surgery with risks of hemodynamic instability such as polytraumatized patients, cranial and cardiac surgery, cesarean delivery for eclampsia, and any other rare instances with no other additional identified triggering factors such as endocrine, hematological, connective tissue disorders, sepsis, renal disease, and chemotherapeutic and immunosuppressant agents.
The second situation, more easily diagnosed, refers to solid organ transplantation (SOT), where other known triggering factors of PRES are identified such as calcineurin inhibitors (CNIs) (cyclosporine A—tacrolimus) [15–19], hemolytic–uremic syndrome (HUS) [20, 21], or hemolytic–uremic syndrome-associated E. coli (HUSEC) involving Shiga toxin-producing E. coli (STEC) [22, 23], both potentially induced by immunosuppressants, sepsis, and renal insufficiency. However, even if use of cyclosporine A and tacrolimus [15–19, 24] is a specific risk factor facilitating occurrence of PRES because of neurotoxicity, with an incidence for cyclosporine A ranging between 0.5% and 35% as reported by M. Hauben [25], review of literature reveals scarce data about other precited factors such as HUS, HUSEC, sepsis, and renal insufficiency. In two remarkable cases [26, 27], an identified risk factor for PRES was sustained hypertension caused by incomplete postoperative pain control. These cases highlight the importance of rigorous management of every step of the surgical course, even something seemingly trivial such as pain control, to avoid severe adverse events such as PRES. Our case is remarkable as it refers to at least three identified factors suspected to trigger PRES, including persistent postinduction hypotension associated with general anesthesia that required vasopressor support, use of cyclosporine A, and HUSEC with STEC, hence complicating therapeutic management.
Nevertheless, our case remains atypical considering characteristics of brain MRI findings, since PRES typically presents with vasogenic edema with lesions symmetrically located in the parieto-occipital regions [28]. In our case, the symmetry was quite constant in the periventricular areas and pulvnar nuclei, while asymmetric hyperintensity was mainly located in the right parieto-occipital region. Actually, several atypical cases of PRES showing asymmetric distribution of hyperintensity signals have been previously reported [29–32].
The pathophysiology of the specific posterior location of PRES still remains largely elusive. Nevertheless, abrupt hemodynamic fluctuations may overcome mechanisms of cerebral autoregulation and hence cause endothelial injury leading to increased blood–brain barrier permeability with transudation of fluid resulting in vasogenic brain edema worsened by damage produced by neuroinflammatory mechanisms involving cytokines, chemokines, oxidative stress, and nitrosative stress [33, 34]. Another pathogenic hypothesis of PRES involves autoregulatory vasoconstriction following hypertension that may lead to brain hypoperfusion and ischemia with a subsequent vasogenic edema [35]. Because the anterior cerebral circulation is much better supplied with sympathetic innervation than the posterior circulation; therefore, the posterior cerebral circulation may be more predisposed to cerebral damage owing to a loss of protective vasoconstriction, breakthrough vasodilation, and vasogenic edema in the face of acute hypertension [36, 37]. In addition, as has been reported [38], if carotid occlusive disease may theoretically protect the brain from hypertensive changes and therefore reduce risk of PRES, it may also result in alteration of vascular autoregulation with cerebral hypoperfusion, ultimately leading to the promotion of a paradoxical occurrence of PRES.
PRES can occur in any age group, in individuals from as young as 2 to 90 years of age [39, 40]. PRES is rare in children, with an incidence of 0.04% [41], and is more frequently encountered in adults, with a slightly increased prevalence in female patients [42]. PRES remains a rare complication following a surgical procedure. Frati et al. [43], in a recent published review of 160 papers, collected 47 patients with PRES following surgery. Mean age of patients was 40.8 years (ages 4–82 years). The most frequent comorbidity reported was cardiovascular disease (14 patients, 29.78%). In 16 patients (36%), no identified risk factors or comorbidities at the time of the surgical procedure could be identified. The most correlated types of surgery were cranial, neuro, and maxillofacial surgery (21 patients, 44.68%) followed by solid organ transplantation (SOT) (8 patients, 17%). Among 47 patients, 2 had undergone heart transplantation (4.25%). Turun Song et al. confirmed [9] rarity of PRES after SOT, since, in a cohort of 4222 patients who underwent SOT from 1998 to 2006, the overall incidence of PRES was 0.49%. However, Navaro et al. [44], in 166 adult patients who received heart transplants over a period of 3 years, reported 4 cases of PRES (2.41%). This result may lead to the suspicion of a tendency to underestimate the real incidence of PRES after SOT, and therefore, awareness of clinicians is crucial to detect organ-transplanted patients with symptoms of PRES. Ultimately, if the overall incidence of PRES diagnosed after surgery is still a source of controversy [45], the incidence of PRES after SOT and especially after heart transplantation (2.41–4.25%) represents a non-neglectable portion of cases and hence deserves special attention to diagnose and treat it in time to ensure full reversibility of symptoms and a benign course in most cases.
PRES is characterized by a large variety of neurological symptoms. Seizure is the most common presentation of PRES in post-transplant recipients (74%), as reported in a cohort of 47 postsurgery patients (49%) in a study by Jeelaniet al. [46] and Frati et al. [43], respectively. Other signs are frequently reported such as visual disturbance (44.68% for Frati; 20% for Jeelani), headache (12.76% for Frati; 26% for Jeelani), and alteration of consciousness (confusion, coma, mental deterioration, cognitive impairment), which is significantly described in 15–28% of the reported cases. Other presentations such as dysarthria, hemiparesis, swallowing deficit, ophthalmoplegia, visual hallucinations, sensory deficits, and vomiting have also been reported [43, 46–48].
It is crucial to note that, if it is impossible to predict the risk of developing PRES after heart transplantation or SOT, it can be a profound help for cardiac surgeons, anesthesiologists, and cardiologists unfamiliar with PRES, in knowing how to recognize it early and treat it in the optimal manner to achieve good clinical outcomes. Our experience is a humble example of unfamiliarity with PRES, since clinical course, adverse events, and symptoms, as well as microbiology and neuroimaging features were retrospectively consistent with an association of HUS and PRES in a context of heart transplantation, supported by two consecutive cerebral MRIs showing white matter abnormalities with hypersignals on the T2/FLAIR sequences and micro bleeds predominately in the occipital, left temporal, and left cerebellum areas. These asymmetric neuroimaging findings were initially assumed to be related with degenerative microvascular leukoencephalopathy. While the neurological situation worsened with a generalized intractable convulsive status epilepticus despite sedation, supported by data of EEG monitoring, concomitant with blood pressure fluctuations and HUS, diagnosis of PRES was finally suspected, necessitating admission to the NICU. Time of onset of PRES presentation varies in patients after SOT. Data from literature reveal that symptoms occur within 2–4 months after organ transplantation, with important disparities in delay ranging from the 3rd postoperative day to the 120th postoperative day [9, 15–17, 19, 24, 41]. Only a few cases presented with PRES later than 1 year after organ transplantation [46]. It is still unclear whether the type of organ transplanted may influence the time of occurrence of PRES. In contrast, it appears that patients who received cyclosporine A had an earlier presentation of PRES compared with those who were given tacrolimus. This likely results from a greater toxicity of cyclosporine A than that of tacrolimus, as suggested by Kochi et al. [49] and Dohgu et al. [50]. In addition, Kochi et al. [51] showed that both cyclosporine A and tacrolimus can induce apoptosis in mouse brain capillary endothelial cells and therefore be partly involved in the occurrence of PRES and other immunosuppressant-induced encephalopathies.
PRES is usually characterized by quite symmetric neuroimaging features. In PRES, neuroanatomic patterns are usually well-known regarding promoting situations, neurological symptoms, and CT scan and MRI findings, which currently display more or less symmetric vasogenic edema of subcortical white matter mainly involving posterior and parietal regions in both hemispheres in 70% of patients and occasionally in the cortex of the occipital and parietal lobes. Frontal lobes may also sporadically be involved [52]. Lesions in other areas, such as cerebellum, brain stem, basal ganglia, spinal cord, or dorsomedial nucleus of the thalamus (pulvinar), are much less commonly reported [53]. In our case, 13 weeks after heart transplantation, MRI showed symmetric abnormalities of both periventricular areas and pulvinars, potentially advocated as unusual locations of PRES while matching with the location of EEG perturbations. At the initial onset of symptoms, non-contrast CT scan may have latency to depict typical lesions of PRES and unusual modality, such as hypoattenuation located in both occipital and parietal lobes, which may be considered primary evidence of PRES characteristics [54]. Nevertheless, literature suggests that MRI is superior to computed tomography in the diagnosis of PRES [52], and our experience retrospectively demonstrated that MRI findings were synchronized with prodromal neurological symptoms of PRES, initially misdiagnosed as a degenerative microvascular leukoencephalopathy. Furthermore, assessing the value of apparent diffusion coefficient (ADC) on brain MRI with FLAIR imaging may be a major determinant to validate diagnosis of atypical PRES. As reported by Matsumoto et al. [55], PRES with only brainstem involvement is rare and characterized by association of reversible hyperintensity on FLAIR imaging with elevated ADC value. In addition, Ninomiya et al. [56] showed that, regardless of diffusion-weighted image (DWI) signal, ADC reduction was associated with a poor neurological outcome.
Therefore, with a higher sensibility, MRI emerged as the most valuable and efficient diagnostic tool as it early displays subcortical hyperintense lesions of the white matter in T2-weighted and FLAIR sequences predominantly in parieto-occipital regions with either a bilateral or unilateral distribution. However, FLAIR sequences remain the appropriate modality rather than conventional T2-weighted spin echo MRI to reveal anatomical details, such as coexistent ischemic and hemorrhagic lesions, and increase resolution of brain edema, as they reduce signals from both the cerebrospinal fluid and contrast between white and gray matter. This modality is particularly interesting for early detection of PRES neuroimaging abnormalities as reported by Turun Song et al. [9]. In addition, MRI is a precious tool, as it offers a better ability to differentiate PRES imaging patterns from other PRES-like pathologies such as white matter diseases such as leukoencephalopathies, which include a wide spectrum of disorders characterized by impairment of normal myelination. Therefore, leukoencephalopthy appears as a main differential diagnosis of PRES with multi-etiological determinants to be considered, such as autoimmune, infectious, vascular, and toxic–metabolic processes, and it is also encountered in patients who underwent SOT. In complex cases with both untypical presentation and MRI patterns, recent advances in imaging potentialities [6] may be useful for a reliable diagnosis of PRES. Hence, magnetic resonance spectroscopy, susceptibility-weighted imaging, positron emission tomography, single-photon emission photography, and fluorodeoxyglucose-positron emission tomography (PET) showing low metabolism in PRES can contribute in helping clinicians to diagnose PRES.
PRES, despite occurring infrequently, deserves to be considered as an important and feared neurologic adverse event following SOT. However, our experience, supported by review of literature, clearly demonstrated that early recognition of both symptoms and neuroimaging patterns as well as identification of trigger factors is a tough challenge. Unfortunately, PRES still remains unfamiliar, and therefore, to prevent neurological sequelae, it is crucial to promote knowledge about this syndrome whose physiopathological mechanisms are not fully elucidated. As previously cited, several factors are known to be involved in endothelial damage and cerebral misperfusion leading to edema detected by MRI or CT scan. These edema imaging patterns mainly result from either vasogenic mechanisms or cytotoxic deleterious consequences. It is now acknowledged that cytotoxic-induced edema is correlated with cyclosporine A and tacrolimus [49–51] independently of the dose. Hence, it is important to identify implicated medications that may lead to permanent neurological sequelae, as Song [9] reported in a review of literature that 89.3% of patients with cyclosporine-associated PRES fully recovered after suppression of cyclosporine medication, while 10.7%, with additional hemorrhagic lesions, had neurological sequelae.
In our patient, blood pressure variations with postinduction hypotension, Shiga toxin-associated hemolytic–uremic syndrome (STEC-HUS), and cyclosporine were retrospectively three identified triggering factors involved in PRES, respectively. Each factor had a specific importance and probably contributed, through synergistic effects, to the initiation of PRES. Postinduction hypotension required both inotropic and vasopressor support, hence leading to abrupt persistent hemodynamic fluctuations with brain misperfusion and unbalanced cerebral autoregulation, causing endothelial injury with disruption of blood–brain barrier integrity resulting in fluid transudation and vasogenic brain edema. Retrospectively, it is very likely that this hemodynamic event played a role in initiating favorable conditions to trigger underlying physiopathogenic mechanisms involved in PRES. The second identified factor was a STEC-HUS diagnosed 6 weeks after heart transplantation, simultaneously with co-occuring neurologic symptoms, while cerebral MRIs showed periventricular diffuse white matter with increased signal intensity on the T2/FLAIR sequences predominately in occipital and left temporal lobes, which were regarded as consistent with degenerative microvascular leukoencephalopathy. Therefore, following recommendations [57, 58], a decision was made to treat STEC-HUS with eculizumab, azithromycin, and apheresis. Despite efficacy of treatment of STEC-HUS, this strategy did not improve the neurological situation, and 2 weeks later, the patient presented altered consciousness, seizures, and coma, necessitating endotracheal intubation and a transfer to the NICU. At last, the third factor identified was administration of cyclosporine despite low residual plasma levels. At this step, the range of clinico-neuroradiological manifestations, along with history of STEC-HUS, hemodynamic instability, and administration of cyclosporine A, led to the diagnosis of PRES and identified cyclosporine A as an active causal neurotoxic factor despite low plasma levels. Therefore, after excluding other known elicitors for PRES or known causes for coma and seizures, the only remaining risk factor was maintenance of immunosuppression therapy with cyclosporine A, and ultimately, diagnosis of cyclosporine-associated PRES was strongly suspected. After interdisciplinary discussions, despite low plasma levels, the decision to discontinue cyclosporine A was validated with no conversion to tacrolimus because of known risks of tacrolimus-induced PRES and HUS [32, 46, 59]. This decision was supported by data from literature, as cyclosporine A-associated neurotoxicity has been reported in up to 40% of patients [60], with a large spectrum of neurological adverse events, including mild tremors, neurocognitive disorders [61], extrapyramidal syndromes [62], and fatal leukoencephalopathy [25].
Actually, the main controversial argument against interruption of cyclosporine A was the low plasma levels, whose neurotoxicity was initially considered as trivial regarding obvious benefits of immunosuppression. However, it is acknowledged that prescription of immunosuppressive drugs such as cyclosporine A or tacrolimus based on polymedications might result in hidden neurotoxicity worsening neurological disorders [63] independently of residual plasma levels, as reported by Asma Danish et al. [64]. Furthermore, direct toxic effects of cyclosporine A on vascular endothelial cells, causing them to release endothelin, prostacyclin, and thromboxane leading to product microthrombi and damage both to the blood–brain barrier and cerebral micro vessels [64] definitely reinforced our decision to interrupt cyclosporine A. Then, considering the risk of causing rejection by discontinuation of cyclosporine A, we opted to switch immunosuppression from cyclosporine A to mycophenolate mofetil, belatacept, and prednisolone, with a prophylaxis treatment for seizures. Within the first days following interruption of cyclosporine A, a steady improvement was observed, and the patient was extubated, progressively recovering, allowing the patient to participate in a physical rehabilitation program.
Conclusions
Despite its relative rarity, we believe that PRES should be systematically considered in a post-heart transplant patient who displays unexplained neurological disorders and receives CNIs. This experience illustrated that knowledge and awareness of clinicians regarding PRES are crucial to detect promoting situations, identify triggering factors, avoid a delay in diagnosis, and hence organize a reactive and optimal therapeutic management to prevent permanent neurological sequelae. Supported by a review of literature, it also highlighted the importance of repeat MRI to facilitate a prompt diagnosis. If PRES imaging is characterized by mostly symmetric hyperintensity signals located in the occipital regions, our case, similar to other published reports, displayed quite frequent asymmetric imaging abnormalities concomitant with symmetric unusual locations, which must not lead to the exclusion of a diagnosis of PRES. At last, our experience obviously demonstrated that PRES is not necessarily a cyclosporine dose-dependent encephalopathy and that summation of promoting and triggering factors may lead to suspect synergistic interactions.
Acknowledgements
The authors would like to thank our colleagues from the NICU (Pitié Salpêtrière) for their support and expertise. The authors dedicate this manuscript to Professor Jean-Pierre Villemot whose contribution and involvement in heart transplantation were crucial throughout the last decades.
Abbreviations
- PRES
Posterior reversible encephalopathy syndrome
- RPLS
Reversible posterior leukoencephalopathy syndrome
- HUS
Hemolytic–uremic syndrome
- STEC
Shiga toxin-producing Escherichia coli
- HUSEC
Hemolytic–uremic syndrome associated Escherichia coli
- ADC
Apparent diffusion coefficient
- FLAIR
Fluid-attenuated inversion recovery
- DWI
Diffusion-weighted image
- EEG
Electroencephalogram
- MRI
Magnetic resonance imaging
- CT scan
Computed tomography scan
- TMA
Thrombotic microangiopathy
- VA-ECMO
Venoarterial extracorporeal membrane oxygenation
- CNI
Calcineurin inhibitor
- SOT
Solid organ transplantation
Author contributions
Daniel Grandmougin was responsible for writing the original draft and revisions; Tristan Ehrlich collected and analyzed the data; Yihua Liu collected and checked the data; Juliette Piccoli collected and analyzed the data; Pan Dan checked the data and the draft; Elodie Phamisith checked the imaging data; Francesco Ferraro collected the data and checked the draft; Christina Sirbu is responsible for the follow-up of transplanted patients; Irina Klemina was in charge of neurological follow-up and EEG analysis; Emmanuelle Schmitt performed and analyzed imaging data; Ismaël Yahia performed and analyzed imaging data; Michael Massin performed and collected echocardiographic data; Benjamin Lefèvre was in charge of the infectious follow-up; Carine Thivilier supervised the therapeutic management in the critical care unit; Fabrice Vanhuyse is responsible for the cardiac assistance program; Stéphane Zuily was our referent for rare diseases and provided his expertise; Juan-Pablo Maureira was responsible for conceptualization and design of the study.
Funding
The authors declare that they did not receive any funding.
Data availability
Data used to support the findings and the conclusions of this manuscript are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The privacy of the patient was considered, and the manuscript does not display any identifying information.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Marra A, Vargas M, Striano P, Del Guercio L, Buonanno P, Servillo G. Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Med Hypotheses. 2014;82(5):619–22. 10.1016/j.mehy.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Ho ML, Rojas R, Eisenberg RL. Cerebral edema. AJR Am J Roentgenol. 2012;199(3):W258–73. 10.2214/AJR.11.8081. [DOI] [PubMed] [Google Scholar]
- 4.Doelken M, Lanz S, Rennert J, Alibek S, Richter G, Doerfler A. Differentiation of cytotoxic and vasogenic edema in a patient with reversible posterior leukoencephalopathy syndrome using diffusion-weighted MRI. Diagn Interv Radiol. 2007;13(3):125–8. [PubMed] [Google Scholar]
- 5.Vanacker P, Matias G, Hagmann P, Michel P. Cerebral hypoperfusion in posterior reversible encephalopathy syndrome is different from transient ischemic attack on CT perfusion. J Neuroimaging. 2015;25(4):643–6. 10.1111/jon.12158. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RC, Patel V, Sheikh-Bahaei N, Liu CSJ, Rajamohan AG, Shiroishi MS, et al. Posterior reversible encephalopathy syndrome (PRES): pathophysiology and neuro-imaging. Front Neurol. 2020;11:463. 10.3389/fneur.2020.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hage P, Kseib C, Hmaimess G, Jaoude PA, Noun P. Recurrent posterior reversible encephalopathy syndrome with cerebellar involvement leading to acute hydrocephalus. Clin Neurol Neurosurg. 2018;172:120–3. 10.1016/j.clineuro.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Ganesh K, Nair RR, Kurian G, Mathew A, Sreedharan S, Paul Z. Posterior reversible encephalopathy syndrome in kidney disease. Kidney Int Rep. 2017 Nov 11;3(2):502-507. 10.1016/j.ekir.2017.10.017. Erratum in: Kidney Int Rep. 2018 Mar 22;3(3):765. PMID: 29725657; PMCID: PMC5932131. [DOI] [PMC free article] [PubMed]
- 9.Song T, Rao Z, Tan Q, Qiu Y, Liu J, Huang Z, et al. Calcineurin inhibitors associated posterior reversible encephalopathy syndrome in solid organ transplantation: report of 2 cases and literature review. Medicine (Baltimore). 2016;95(14):e3173. 10.1097/MD.0000000000003173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914–25. 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 11.Feske SK. Posterior reversible encephalopathy syndrome: a review. Semin Neurol. 2011;31(2):202–15. 10.1055/s-0031-1277990. [DOI] [PubMed] [Google Scholar]
- 12.Balcerac A, Bihan K, Psimaras D, Lebrun-Vignes B, Salem JE, Weiss N. Drugs associated with posterior reversible encephalopathy syndrome, a worldwide signal detection study. J Neurol. 2023;270(2):975–85. 10.1007/s00415-022-11450-y. [DOI] [PubMed] [Google Scholar]
- 13.Triquenot-Bagan A, Gerardin E, Guegan-Massardier E, Onnient Y, Leroy F, Mihout B. Postoperative reversible posterior leukoencephalopathy syndrome. Cerebrovasc Dis. 2003;16(4):430–2. 10.1159/000072568. [DOI] [PubMed] [Google Scholar]
- 14.Zimering JH, Mesfin A. Posterior reversible encephalopathy syndrome following elevated mean arterial pressures for cervical spinal cord injury. J Spinal Cord Med. 2018;41(1):111–4. 10.1080/10790268.2016.1250030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akutsu N, Iwashita C, Maruyama M, Ootsuki K, Ito T, Saigo K, et al. Two cases of calcineurin inhibitor-associated reversible posterior leukoencephalopathy syndrome in renal transplant recipients. Transplant Proc. 2008;40(7):2416–8. 10.1016/j.transproceed.2008.07.104. [DOI] [PubMed] [Google Scholar]
- 16.Huenges K, Kolat P, Panholzer B, Haneya A. CSA-induced PRES after heart transplantation-report of two cases and review. Thorac Cardiovasc Surg Rep. 2021;10(1):e59–60. 10.1055/s-0041-1732344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oda N, Kato TS, Hanatani A, Niwaya K, Nakatani T, Ishibashi-Ueda H, et al. Reversible posterior leukoencephalopathy syndrome (RPLS) in a heart transplant recipient treated by substitution of cyclosporine A with tacrolimus. Intern Med. 2010;49(11):1013–6. 10.2169/internalmedicine.49.3012. [DOI] [PubMed] [Google Scholar]
- 18.Bloom J, Collins ML, Belovsky MP, Feduska E, Schofield P, Leong R, et al. Perfusion-dependent focal neurologic deficits in a critically ill heart transplant recipient: a case of tacrolimus-associated reversible cerebral vasospasm syndrome? J Cardiothorac Vasc Anesth. 2023;37(8):1487–94. 10.1053/j.jvca.2023.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez R, Muskula PR, Everley MP. Posterior reversible encephalopathy syndrome after orthotopic heart transplantation: a case report. Am J Case Rep. 2017;18:487–90. 10.12659/ajcr.903403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weil EL, Rabinstein AA. Neurological manifestations of thrombotic microangiopathy syndromes in adult patients. J Thromb Thrombolysis. 2021;51(4):1163–9. 10.1007/s11239-021-02431-5. [DOI] [PubMed] [Google Scholar]
- 21.Gómez-Lado C, Martinón-Torres F, Alvarez-Moreno A, Eirís-Puñal J, Carreira-Sande N, Rodriguez-Nuñez A, et al. Leucoencefalopatía posterior reversible: una complicación infrecuente en el curso del síndrome hemolítico urémico [Reversible posterior leukoencephalopathy syndrome: an infrequent complication in the course of haemolytic–uremic syndrome]. Rev Neurol. 2007;44(8):475–8. [PubMed] [Google Scholar]
- 22.Khalid M, Andreoli S. Extrarenal manifestations of the hemolytic uremic syndrome associated with Shiga toxin-producing Escherichia coli (STEC HUS). Pediatr Nephrol. 2019;34(12):2495–507. 10.1007/s00467-018-4105-1. [DOI] [PubMed] [Google Scholar]
- 23.Costigan C, Raftery T, Carroll AG, Wildes D, Reynolds C, Cunney R, et al. Neurological involvement in children with hemolytic uremic syndrome. Eur J Pediatr. 2022;181(2):501–12. 10.1007/s00431-021-04200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loar RW, Patterson MC, O’Leary PW, Driscoll DJ, Johnson JN. Posterior reversible encephalopathy syndrome and hemorrhage associated with tacrolimus in a pediatric heart transplantation recipient. Pediatr Transplant. 2013;17(2):E67-70. 10.1111/petr.12039. [DOI] [PubMed] [Google Scholar]
- 25.Hauben M. Cyclosporine neurotoxicity. Pharmacotherapy. 1996;16(4):576–83. [PubMed] [Google Scholar]
- 26.Yi JH, Ha SH, Kim YK, Choi EM. Posterior reversible encephalopathy syndrome in an untreated hypertensive patient after spinal surgery under general anesthesia—A case report-. Korean J Anesthesiol. 2011;60(5):369–72. 10.4097/kjae.2011.60.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato N, Machida H, Kodaka M, Nishiyama K, Komori M. Perioperative posterior reversible encephalopathy syndrome in a patient with no history of hypertension: a case report. JA Clin Rep. 2016;2(1):38. 10.1186/s40981-016-0065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tetsuka S, Ogawa T. Posterior reversible encephalopathy syndrome: a review with emphasis on neuroimaging characteristics. J Neurol Sci. 2019;404:72–9. 10.1016/j.jns.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Cerrone P, Sucapane P, Totaro R, Sacco S, Carolei A, Marini C. Posterior reversible encephalopathy syndrome presenting with atypical findings: report of two cases. Case Rep Neurol Med. 2018;2018:7835415. 10.1155/2018/7835415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rona G, Arifoğlu M, Günbey HP, Yükselmiş U. Influenza A (H1N1)-associated acute necrotizing encephalopathy with unusual posterior reversible encephalopathy syndrome in a child. SN Compr Clin Med. 2021;3(7):1528–33. 10.1007/s42399-021-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An JY, Lee YH, Kim W. Asymmetric posterior reversible encephalopathy syndrome mimicking massive cerebral hemispheric infarction after cardiac surgery. Neurol India. 2021;69(6):1621–2. 10.4103/0028-3886.333490. [DOI] [PubMed] [Google Scholar]
- 32.Kommana SS, Bains U, Fasula V, Henderer J. A case of Tacrolimus-induced posterior reversible encephalopathy syndrome initially presenting as a bilateral optic neuropathy. Case Rep Ophthalmol. 2019;10(1):140–4. 10.1159/000496916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang C, Hawkins KE, Doré S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316(2):C135–53. 10.1152/ajpcell.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horbinski C, Bartynski WS, Carson-Walter E, Hamilton RL, Tan HP, Cheng S. Reversible encephalopathy after cardiac transplantation: histologic evidence of endothelial activation, T-cell specific trafficking, and vascular endothelial growth factor expression. AJNR Am J Neuroradiol. 2009;30(3):588–90. 10.3174/ajnr.A1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapoor A, Birks E, Lenneman A, McCants K. Posterior reversible encephalopathy syndrome after heart transplantation: diagnosis and immunosuppressive therapy. Tex Heart Inst J. 2017;44(3):205–8. 10.14503/THIJ-15-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brubaker LM, Smith JK, Lee YZ, Lin W, Castillo M. Hemodynamic and permeability changes in posterior reversible encephalopathy syndrome measured by dynamic susceptibility perfusion-weighted MR imaging. AJNR Am J Neuroradiol. 2005;26(4):825–30. [PMC free article] [PubMed] [Google Scholar]
- 37.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043–9. 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kühn AL, Siddalingappa A, Chang YM, Bhadelia R. The effect of carotid occlusive disease on the distribution of brain lesions in patients with systemic conditions-an example of PRES. Radiol Case Rep. 2019;14(9):1136–9. 10.1016/j.radcr.2019.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23(6):1038–48. [PMC free article] [PubMed] [Google Scholar]
- 40.Habetz K, Ramakrishnaiah R, Raina SK, Fitzgerald RT, Hinduja A. Posterior reversible encephalopathy syndrome: a comparative study of pediatric versus adult patients. Pediatr Neurol. 2016;65:45–51. 10.1016/j.pediatrneurol.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Das BB, Ghaleb S, Moskowitz W, Arya S, Taylor M. Posterior reversible encephalopathy syndrome in a pediatric heart transplant recipient with coarctation of aorta. Ann Pediatr Cardiol. 2022;15(5–6):518–22. 10.4103/apc.apc_235_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamy C, Oppenheim C, Mas JL. Posterior reversible encephalopathy syndrome. Handb Clin Neurol. 2014;121:1687–701. 10.1016/B978-0-7020-4088-7.00109-7. [DOI] [PubMed] [Google Scholar]
- 43.Frati A, Armocida D, Tartara F, Cofano F, Corvino S, Paolini S, et al. Can post-operative posterior reversible encephalopathy syndrome (PRES) be considered an insidious rare surgical complication? Brain Sci. 2023;13(5): 706. 10.3390/brainsci13050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navarro V, Varnous S, Galanaud D, Vaissier E, Granger B, Gandjbakhch I, et al. Incidence and risk factors for seizures after heart transplantation. J Neurol. 2010;257(4):563–8. 10.1007/s00415-009-5366-1. [DOI] [PubMed] [Google Scholar]
- 45.Apuri S, Carlin K, Bass E, Nguyen PT, Greene JN. Tacrolimus associated posterior reversible encephalopathy syndrome - a case series and review. Mediterr J Hematol Infect Dis. 2014;6(1):e2014014. 10.4084/MJHID.2014.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeelani H, Sharma A, Halawa AM. Posterior reversible encephalopathy syndrome in organ transplantation. Exp Clin Transplant. 2022;20(7):642–8. 10.6002/ect.2021.0268. [DOI] [PubMed] [Google Scholar]
- 47.Kuhnt D, Becker A, Benes L, Nimsky C. Reversible cortical blindness and internuclear ophthalmoplegia after neurosurgical operation: case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2013;74:e128–32. 10.1055/s-0032-1327448. [DOI] [PubMed] [Google Scholar]
- 48.Hansberry DR, Agarwal N, Tomei KL, Goldstein IM. Posterior reversible encephalopathy syndrome in a patient with a Chiari I malformation. Surg Neurol Int. 2013;4:130. 10.4103/2152-7806.119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kochi S, Takanaga H, Matsuo H, Naito M, Tsuruo T, Sawada Y. Effect of cyclosporin A or tacrolimus on the function of blood-brain barrier cells. Eur J Pharmacol. 1999;372(3):287–95. 10.1016/s0014-2999(99)00247-2. [DOI] [PubMed] [Google Scholar]
- 50.Dohgu S, Kataoka Y, Ikesue H, Naito M, Tsuruo T, Oishi R, et al. Involvement of glial cells in cyclosporine-increased permeability of brain endothelial cells. Cell Mol Neurobiol. 2000;20(6):781–6. 10.1023/a:1007015228318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kochi S, Takanaga H, Matsuo H, Ohtani H, Naito M, Tsuruo T, et al. Induction of apoptosis in mouse brain capillary endothelial cells by cyclosporin A and tacrolimus. Life Sci. 2000;66(23):2255–60. 10.1016/s0024-3205(00)00554-3. [DOI] [PubMed] [Google Scholar]
- 52.Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28(7):1320–7. 10.3174/ajnr.A0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ou S, Xia L, Wang L, Xia L, Zhou Q, Pan S. Posterior reversible encephalopathy syndrome with isolated involving infratentorial structures. Front Neurol. 2018;9:843. 10.3389/fneur.2018.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dandoy CE, Linscott LL, Davies SM, Leach JL, Myers KC, El-Bietar J, et al. Clinical utility of computed tomography and magnetic resonance imaging for diagnosis of posterior reversible encephalopathy syndrome after stem cell transplantation in children and adolescents. Biol Blood Marrow Transplant. 2015;21(11):2028–32. 10.1016/j.bbmt.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumoto N, Ogawa T, Hishikawa N, Takao Y, Fujii S. Dramatic amelioration in serial magnetic resonance imaging in an “isolated brainstem” reversible encephalopathy syndrome case. Yonago Acta Med. 2023;66(2):297–9. 10.33160/yam.2023.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ninomiya I, Kanazawa M, Akaiwa Y, Shimohata T, Okamoto K, Onodera O, et al. Apparent diffusion coefficient reduction might be a predictor of poor outcome in patients with posterior reversible encephalopathy syndrome. J Neurol Sci. 2017;381:1–3. 10.1016/j.jns.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Mahat U, Matar RB, Rotz SJ. Use of complement monoclonal antibody eculizumab in Shiga toxin producing Escherichia coli associated hemolytic uremic syndrome: a review of current evidence. Pediatr Blood Cancer. 2019;66(11):e27913. 10.1002/pbc.27913. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Thaker H, Wang C, Xu Z, Dong M. Diagnosis and treatment for Shiga toxin-producing Escherichia coli associated hemolytic uremic syndrome. Toxins (Basel). 2022;15(1):10. 10.3390/toxins15010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaur G, Ashraf I, Peck MM, Maram R, Mohamed A, Ochoa Crespo D, et al. Chemotherapy and immunosuppressant therapy-induced posterior reversible encephalopathy syndrome. Cureus. 2020;12(10):e11163. 10.7759/cureus.11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gijtenbeek JMM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: a review. J Neurol. 1999;246:339–46. 10.1007/s004150050360. [DOI] [PubMed] [Google Scholar]
- 61.Thiankhaw K, Chattipakorn N, Chattipakorn SC. How calcineurin inhibitors affect cognition. Acta Physiol (Oxf). 2024;240(7):e14161. 10.1111/apha.14161. [DOI] [PubMed] [Google Scholar]
- 62.Al-Amrani F, Al Rawas A, Al-Ajmi E, Al Futaisi A. Case report: cyclosporine A-induced extrapyramidal syndrome following hematopoietic stem cell transplantation. Front Neurol. 2023;14:1140732. 10.3389/fneur.2023.1140732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tolou-Ghamari Z. Tacrolimus and cyclosporin pharmacotherapy, detection methods, cytochrome P450 enzymes after heart transplantation. Cardiovasc Hematol Agents Med Chem. 2024;22(2):106–13. 10.2174/1871525721666230726150021. [DOI] [PubMed] [Google Scholar]
- 64.Danish A, Mughal SI, Zaidi U, Dildar S, Samad S, Jamal A, et al. Frequency and risk factors of cyclosporine-induced neurotoxicity in allogeneic stem cell transplant recipients. Cureus. 2021;13(11):e19824. 10.7759/cureus.19824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to support the findings and the conclusions of this manuscript are available from the corresponding author upon request.


