Abstract
The interaction of a nonspecific wheat lipid transfer protein (LTP) with phospholipids has been studied using the monolayer technique as a simplified model of biological membranes. The molecular organization of the LTP-phospholipid monolayer has been determined by using polarized attenuated total internal reflectance infrared spectroscopy, and detailed information on the microstructure of the mixed films has been investigated by using epifluorescence microscopy. The results show that the incorporation of wheat LTP within the lipid monolayers is surface-pressure dependent. When LTP is injected into the subphase under a dipalmytoylphosphatidylglycerol monolayer at low surface pressure (< 20 mN/m), insertion of the protein within the lipid monolayer leads to an expansion of dipalmytoylphosphatidylglycerol surface area. This incorporation leads to a decrease in the conformational order of the lipid acyl chains and results in an increase in the size of the solid lipid domains, suggesting that LTP penetrates both expanded and solid domains. By contrast, when the protein is injected under the lipid at high surface pressure (> or = 20 mN/m) the presence of LTP leads neither to an increase of molecular area nor to a change of the lipid order, even though some protein molecules are bound to the surface of the monolayer, which leads to an increase of the exposure of the lipid ester groups to the aqueous environment. On the other hand, the conformation of LTP, as well as the orientation of alpha-helices, is surface-pressure dependent. At low surface pressure, the alpha-helices inserted into the monolayers are rather parallel to the monolayer plane. In contrast, at high surface pressure, the alpha-helices bound to the surface of the monolayers are neither parallel nor perpendicular to the interface but in an oblique orientation.
Full text
PDF


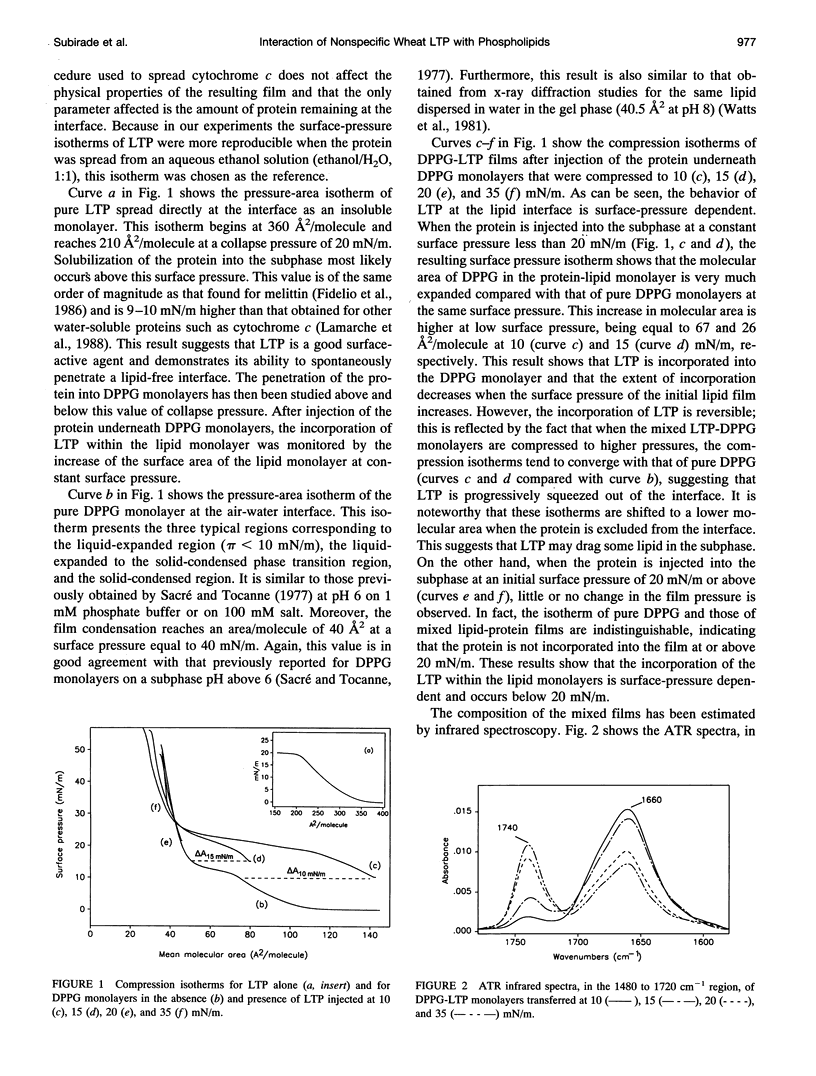






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlers M., Grainger D. W., Herron J. N., Lim K., Ringsdorf H., Salesse C. Quenching of fluorescein-conjugated lipids by antibodies. Quantitative recognition and binding of lipid-bound haptens in biomembrane models, formation of two-dimensional protein domains and molecular dynamics simulations. Biophys J. 1992 Sep;63(3):823–838. doi: 10.1016/S0006-3495(92)81645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrondo J. L., Muga A., Castresana J., Goñi F. M. Quantitative studies of the structure of proteins in solution by Fourier-transform infrared spectroscopy. Prog Biophys Mol Biol. 1993;59(1):23–56. doi: 10.1016/0079-6107(93)90006-6. [DOI] [PubMed] [Google Scholar]
- Babin Y., D'Amour J., Pigeon M., Pézolet M. A study of the structure of polymyxin B-dipalmitoylphosphatidylglycerol complexes by vibrational spectroscopy. Biochim Biophys Acta. 1987 Sep 18;903(1):78–88. doi: 10.1016/0005-2736(87)90157-x. [DOI] [PubMed] [Google Scholar]
- Billheimer J. T., Gaylor J. L. Effect of lipid composition on the transfer of sterols mediated by non-specific lipid transfer protein (sterol carrier protein2). Biochim Biophys Acta. 1990 Sep 18;1046(2):136–143. doi: 10.1016/0005-2760(90)90180-6. [DOI] [PubMed] [Google Scholar]
- Blankenburg R., Meller P., Ringsdorf H., Salesse C. Interaction between biotin lipids and streptavidin in monolayers: formation of oriented two-dimensional protein domains induced by surface recognition. Biochemistry. 1989 Oct 3;28(20):8214–8221. doi: 10.1021/bi00446a037. [DOI] [PubMed] [Google Scholar]
- Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C = O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988 Oct 18;27(21):8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- Bougis P., Rochat H., Piéroni G., Verger R. Penetration of phospholipid monolayers by cardiotoxins. Biochemistry. 1981 Aug 18;20(17):4915–4920. doi: 10.1021/bi00520a017. [DOI] [PubMed] [Google Scholar]
- Brauner J. W., Mendelsohn R., Prendergast F. G. Attenuated total reflectance Fourier transform infrared studies of the interaction of melittin, two fragments of melittin, and delta-hemolysin with phosphatidylcholines. Biochemistry. 1987 Dec 15;26(25):8151–8158. doi: 10.1021/bi00399a020. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Casal H. L., Mantsch H. H. Characterization of the pretransition in 1,2-dipalmitoyl-sn-glycero-3-phosphocholine by Fourier transform infrared spectroscopy. Biochemistry. 1980 Aug 5;19(16):3665–3672. doi: 10.1021/bi00557a005. [DOI] [PubMed] [Google Scholar]
- Carrier D., Pézolet M. Investigation of polylysine-dipalmitoylphosphatidylglycerol interactions in model membranes. Biochemistry. 1986 Jul 15;25(14):4167–4174. doi: 10.1021/bi00362a027. [DOI] [PubMed] [Google Scholar]
- Casal H. L., Mantsch H. H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim Biophys Acta. 1984 Dec 4;779(4):381–401. doi: 10.1016/0304-4157(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Cornell D. G., Dluhy R. A., Briggs M. S., McKnight C. J., Gierasch L. M. Conformations and orientations of a signal peptide interacting with phospholipid monolayers. Biochemistry. 1989 Apr 4;28(7):2789–2797. doi: 10.1021/bi00433a008. [DOI] [PubMed] [Google Scholar]
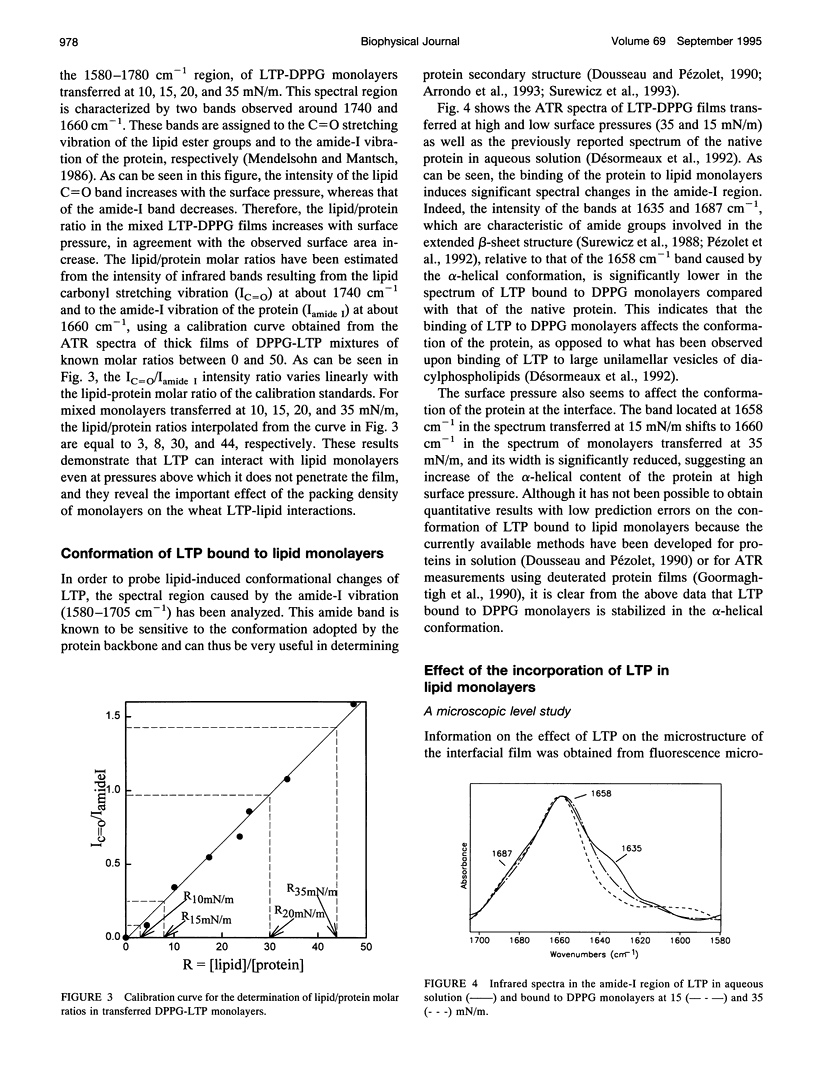
- Dousseau F., Pézolet M. Determination of the secondary structure content of proteins in aqueous solutions from their amide I and amide II infrared bands. Comparison between classical and partial least-squares methods. Biochemistry. 1990 Sep 18;29(37):8771–8779. doi: 10.1021/bi00489a038. [DOI] [PubMed] [Google Scholar]
- Draper R. P., Waterfield C. J., York M. J., Timbrell J. A. Studies on the muscle toxicant 2,3,5,6-tetramethyl p-phenylenediamine: effects on various biomarkers including urinary creatine and taurine. Arch Toxicol. 1994;69(2):111–117. doi: 10.1007/s002040050145. [DOI] [PubMed] [Google Scholar]
- Désormeaux A., Blochet J. E., Pézolet M., Marion D. Amino acid sequence of a non-specific wheat phospholipid transfer protein and its conformation as revealed by infrared and Raman spectroscopy. Role of disulfide bridges and phospholipids in the stabilization of the alpha-helix structure. Biochim Biophys Acta. 1992 May 22;1121(1-2):137–152. doi: 10.1016/0167-4838(92)90347-g. [DOI] [PubMed] [Google Scholar]
- Fattal D. R., Ben-Shaul A. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys J. 1993 Nov;65(5):1795–1809. doi: 10.1016/S0006-3495(93)81249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S., Tamm L. K. Orientation of melittin in phospholipid bilayers. A polarized attenuated total reflection infrared study. Biophys J. 1991 Oct;60(4):922–930. doi: 10.1016/S0006-3495(91)82126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldwerth D., de Kermel A., Zachowski A., Guerbette F., Kader J. C., Henry J. P., Devaux P. F. Use of spin-labeled and fluorescent lipids to study the activity of the phospholipid transfer protein from maize seedlings. Biochim Biophys Acta. 1991 Apr 3;1082(3):255–264. doi: 10.1016/0005-2760(91)90201-r. [DOI] [PubMed] [Google Scholar]
- Gincel E., Simorre J. P., Caille A., Marion D., Ptak M., Vovelle F. Three-dimensional structure in solution of a wheat lipid-transfer protein from multidimensional 1H-NMR data. A new folding for lipid carriers. Eur J Biochem. 1994 Dec 1;226(2):413–422. doi: 10.1111/j.1432-1033.1994.tb20066.x. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Cabiaux V., Ruysschaert J. M. Secondary structure and dosage of soluble and membrane proteins by attenuated total reflection Fourier-transform infrared spectroscopy on hydrated films. Eur J Biochem. 1990 Oct 24;193(2):409–420. doi: 10.1111/j.1432-1033.1990.tb19354.x. [DOI] [PubMed] [Google Scholar]
- Heckl W. M., Zaba B. N., Möhwald H. Interactions of cytochromes b5 and c with phospholipid monolayers. Biochim Biophys Acta. 1987 Sep 18;903(1):166–176. doi: 10.1016/0005-2736(87)90166-0. [DOI] [PubMed] [Google Scholar]
- Hübner W., Mantsch H. H. Orientation of specifically 13C=O labeled phosphatidylcholine multilayers from polarized attenuated total reflection FT-IR spectroscopy. Biophys J. 1991 Jun;59(6):1261–1272. doi: 10.1016/S0006-3495(91)82341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner W., Mantsch H. H., Paltauf F., Hauser H. Conformation of phosphatidylserine in bilayers as studied by Fourier transform infrared spectroscopy. Biochemistry. 1994 Jan 11;33(1):320–326. doi: 10.1021/bi00167a042. [DOI] [PubMed] [Google Scholar]
- Johnson S. J., Bayerl T. M., Weihan W., Noack H., Penfold J., Thomas R. K., Kanellas D., Rennie A. R., Sackmann E. Coupling of spectrin and polylysine to phospholipid monolayers studied by specular reflection of neutrons. Biophys J. 1991 Nov;60(5):1017–1025. doi: 10.1016/S0006-3495(91)82139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen P. K., Kõiv A., Lehtonen J. Y., Rytömaa M., Mustonen P. Lipid dynamics and peripheral interactions of proteins with membrane surfaces. Chem Phys Lipids. 1994 Sep 6;73(1-2):181–207. doi: 10.1016/0009-3084(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Samson I., Pézolet M. Investigation of the interaction between melittin and dipalmitoylphosphatidylglycerol bilayers by vibrational spectroscopy. Chem Phys Lipids. 1991 Oct;59(3):233–244. doi: 10.1016/0009-3084(91)90023-5. [DOI] [PubMed] [Google Scholar]
- Molina A., Segura A., García-Olmedo F. Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett. 1993 Jan 25;316(2):119–122. doi: 10.1016/0014-5793(93)81198-9. [DOI] [PubMed] [Google Scholar]
- Mombers C., de Gier J., Demel R. A., van Deenen L. L. Spectrin-phospholipid interaction. A monolayer study. Biochim Biophys Acta. 1980 Dec 2;603(1):52–62. doi: 10.1016/0005-2736(80)90390-9. [DOI] [PubMed] [Google Scholar]
- Nargessi R. D., Smith D. S. Fluorometric assays for avidin and biotin. Methods Enzymol. 1986;122:67–72. doi: 10.1016/0076-6879(86)22150-3. [DOI] [PubMed] [Google Scholar]
- Pascher I., Sundell S., Harlos K., Eibl H. Conformation and packing properties of membrane lipids: the crystal structure of sodium dimyristoylphosphatidylglycerol. Biochim Biophys Acta. 1987 Jan 9;896(1):77–88. doi: 10.1016/0005-2736(87)90358-0. [DOI] [PubMed] [Google Scholar]
- Pebay-Peyroula E., Cohen-Addad C., Lehmann M. S., Marion D. Crystallographic data for the 9000 dalton wheat non-specific phospholipid transfer protein. J Mol Biol. 1992 Jul 20;226(2):563–564. doi: 10.1016/0022-2836(92)90970-u. [DOI] [PubMed] [Google Scholar]
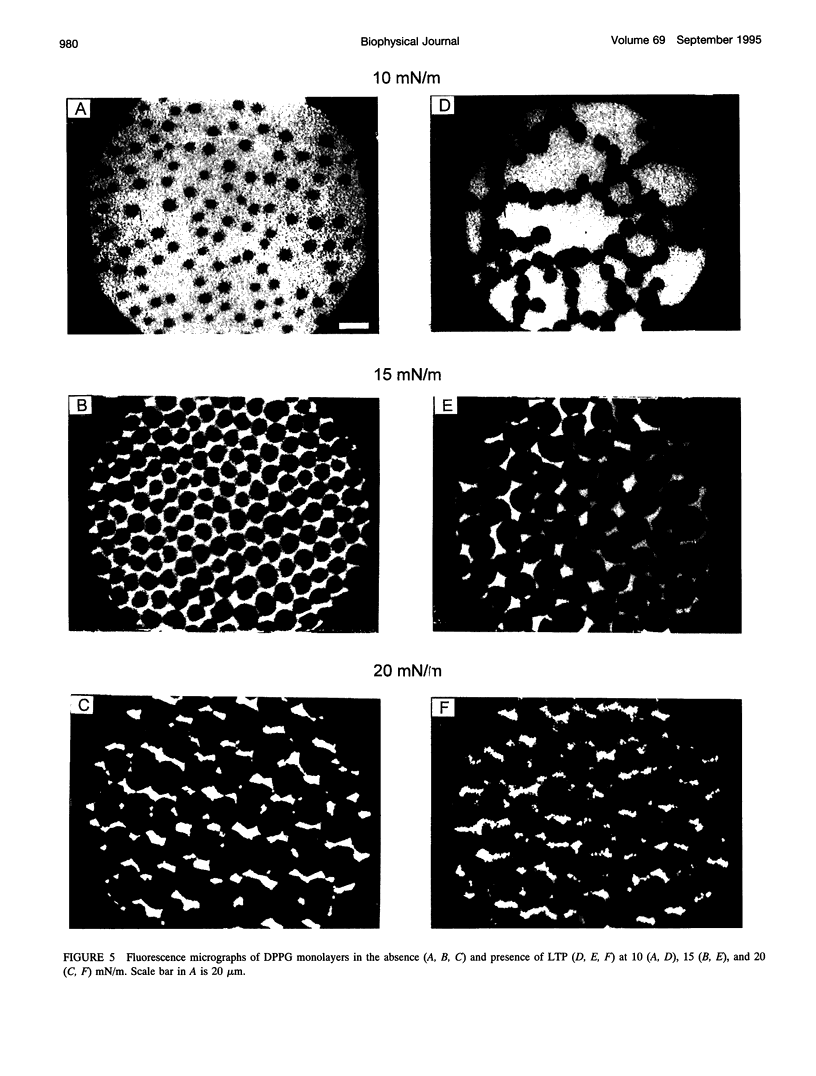
- Peters R., Beck K. Translational diffusion in phospholipid monolayers measured by fluorescence microphotolysis. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7183–7187. doi: 10.1073/pnas.80.23.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit M. C., Sodano P., Marion D., Ptak M. Two-dimensional 1H-NMR studies of maize lipid-transfer protein. Sequence-specific assignment and secondary structure. Eur J Biochem. 1994 Jun 15;222(3):1047–1054. doi: 10.1111/j.1432-1033.1994.tb18957.x. [DOI] [PubMed] [Google Scholar]
- Pézolet M., Bonenfant S., Dousseau F., Popineau Y. Conformation of wheat gluten proteins. Comparison between functional and solution states as determined by infrared spectroscopy. FEBS Lett. 1992 Mar 16;299(3):247–250. doi: 10.1016/0014-5793(92)80125-z. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacré M. M., Tocanne J. F. Importance of glycerol and fatty acid residues on the ionic properties of phosphatidylglycerols at the air-water interface. Chem Phys Lipids. 1977 Apr;18(3-4):334–354. doi: 10.1016/0009-3084(77)90019-6. [DOI] [PubMed] [Google Scholar]
- Shin D. H., Lee J. Y., Hwang K. Y., Kim K. K., Suh S. W. High-resolution crystal structure of the non-specific lipid-transfer protein from maize seedlings. Structure. 1995 Feb 15;3(2):189–199. doi: 10.1016/s0969-2126(01)00149-6. [DOI] [PubMed] [Google Scholar]
- Simorre J. P., Caille A., Marion D., Marion D., Ptak M. Two- and three-dimensional 1H NMR studies of a wheat phospholipid transfer protein: sequential resonance assignments and secondary structure. Biochemistry. 1991 Dec 10;30(49):11600–11608. doi: 10.1021/bi00113a016. [DOI] [PubMed] [Google Scholar]
- Sossountzov L., Ruiz-Avila L., Vignols F., Jolliot A., Arondel V., Tchang F., Grosbois M., Guerbette F., Miginiac E., Delseny M. Spatial and temporal expression of a maize lipid transfer protein gene. Plant Cell. 1991 Sep;3(9):923–933. doi: 10.1105/tpc.3.9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
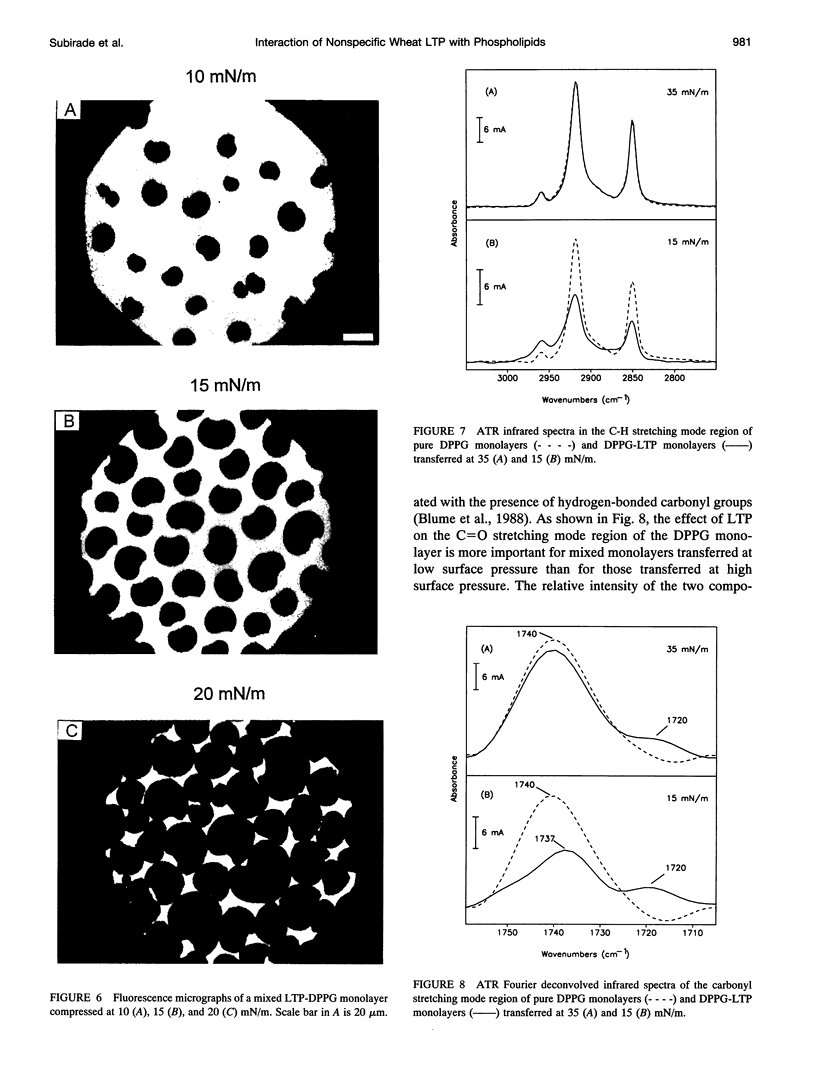
- Sterk P., Booij H., Schellekens G. A., Van Kammen A., De Vries S. C. Cell-specific expression of the carrot EP2 lipid transfer protein gene. Plant Cell. 1991 Sep;3(9):907–921. doi: 10.1105/tpc.3.9.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H., Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry. 1993 Jan 19;32(2):389–394. doi: 10.1021/bi00053a001. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim Biophys Acta. 1988 Jan 29;952(2):115–130. doi: 10.1016/0167-4838(88)90107-0. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Moscarello M. A., Mantsch H. H. Fourier transform infrared spectroscopic investigation of the interaction between myelin basic protein and dimyristoylphosphatidylglycerol bilayers. Biochemistry. 1987 Jun 30;26(13):3881–3886. doi: 10.1021/bi00387a021. [DOI] [PubMed] [Google Scholar]
- Tchang F., This P., Stiefel V., Arondel V., Morch M. D., Pages M., Puigdomenech P., Grellet F., Delseny M., Bouillon P. Phospholipid transfer protein: full-length cDNA and amino acid sequence in maize. Amino acid sequence homologies between plant phospholipid transfer proteins. J Biol Chem. 1988 Nov 15;263(32):16849–16855. [PubMed] [Google Scholar]
- Teissie J. Interaction of cytochrome c with phospholipid monolayers. Orientation and penetration of protein as functions of the packing density of film, nature of the phospholipids, and ionic content of the aqueous phase. Biochemistry. 1981 Mar 17;20(6):1554–1560. doi: 10.1021/bi00509a023. [DOI] [PubMed] [Google Scholar]
- Terras F. R., Goderis I. J., Van Leuven F., Vanderleyden J., Cammue B. P., Broekaert W. F. In Vitro Antifungal Activity of a Radish (Raphanus sativus L.) Seed Protein Homologous to Nonspecific Lipid Transfer Proteins. Plant Physiol. 1992 Oct;100(2):1055–1058. doi: 10.1104/pp.100.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura J., Cameron D. G., Mantsch H. H. A Fourier transform infrared spectroscopic study of the molecular interaction of cholesterol with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine. Biochim Biophys Acta. 1980 Oct 16;602(1):32–44. doi: 10.1016/0005-2736(80)90287-4. [DOI] [PubMed] [Google Scholar]
- Watts A., Harlos K., Marsh D. Charge-induced tilt in ordered-phase phosphatidylglycerol bilayers evidence from X-ray diffraction. Biochim Biophys Acta. 1981 Jul 6;645(1):91–96. doi: 10.1016/0005-2736(81)90515-0. [DOI] [PubMed] [Google Scholar]
- Weis R. M. Fluorescence microscopy of phospholipid monolayer phase transitions. Chem Phys Lipids. 1991 Mar;57(2-3):227–239. doi: 10.1016/0009-3084(91)90078-p. [DOI] [PubMed] [Google Scholar]
- Weis R. M., McConnell H. M. Two-dimensional chiral crystals of phospholipid. Nature. 1984 Jul 5;310(5972):47–49. doi: 10.1038/310047a0. [DOI] [PubMed] [Google Scholar]
- Zull J. E., Greanoff S., Adam H. K. Interaction of egg lecithin with cholesterol in the solid state. Biochemistry. 1968 Dec;7(12):4172–4176. doi: 10.1021/bi00852a005. [DOI] [PubMed] [Google Scholar]
- van Amerongen A., Demel R. A., Westerman J., Wirtz K. W. Transfer of cholesterol and oxysterol derivatives by the nonspecific lipid transfer protein (sterol carrier protein 2): a study on its mode of action. Biochim Biophys Acta. 1989 Jul 17;1004(1):36–43. doi: 10.1016/0005-2760(89)90209-9. [DOI] [PubMed] [Google Scholar]





