Abstract
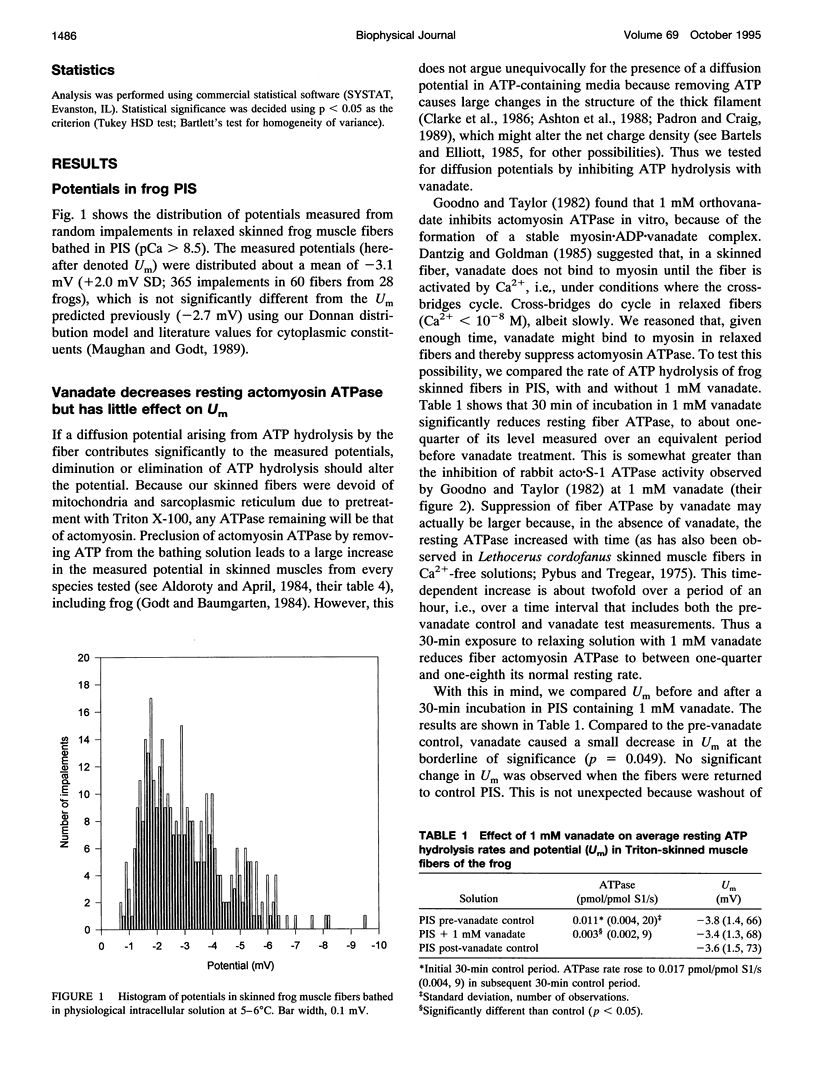
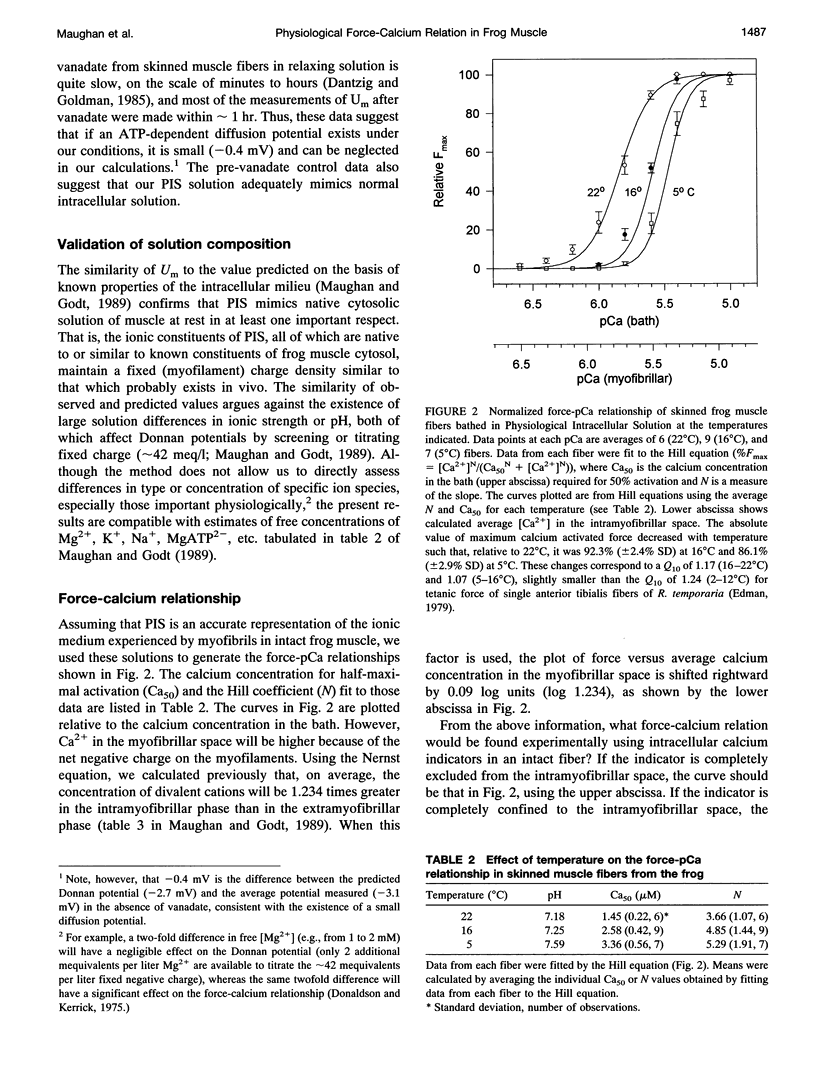
In previous papers we used estimates of the composition of frog muscle and calculations involving the likely fixed charge density in myofibrils to propose bathing solutions for skinned fibers, which best mimic the normal intracellular milieu of intact muscle fibers. We tested predictions of this calculation using measurements of the potential across the boundary of skinned frog muscle fibers bathed in this solution. The average potential was -3.1 mV, close to that predicted from a simple Donnan equilibrium. The contribution of ATP hydrolysis to a diffusion potential was probably small because addition of 1 mM vanadate to the solution decreased the fiber actomyosin ATPase rate (measured by high-performance liquid chromatography) by at least 73% but had little effect on the measured potential. Using these solutions, we obtained force-pCa curves from mechanically skinned fibers at three different temperatures, allowing the solution pH to change with temperature in the same fashion as the intracellular pH of intact fibers varies with temperature. The bath concentration of Ca2+ required for half-maximal activation of isometric force was 1.45 microM (22 degrees C, pH 7.18), 2.58 microM (16 degrees C, pH 7.25), and 3.36 microM (5 degrees C, pH 7.59). The [Ca2+] at the threshold of activation at 16 degrees C was approximately 1 microM, in good agreement with estimates of threshold [Ca2+] in intact frog muscle fibers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldoroty R. A., April E. W. Donnan potentials from striated muscle liquid crystals. A-band and I-band measurements. Biophys J. 1984 Dec;46(6):769–779. doi: 10.1016/S0006-3495(84)84075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldoroty R. A., Garty N. B., April E. W. Donnan potentials from striated muscle liquid crystals. Lattice spacing dependence. Biophys J. 1987 Mar;51(3):371–381. doi: 10.1016/S0006-3495(87)83359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Lee J. A., Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989 Aug;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E. M., Elliott G. F. Donnan potentials from the A- and I-bands of glycerinated and chemically skinned muscles, relaxed and in rigor. Biophys J. 1985 Jul;48(1):61–76. doi: 10.1016/S0006-3495(85)83760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M. L., Hofman W., Wray J. S. ATP binding and crossbridge structure in muscle. J Mol Biol. 1986 Oct 5;191(3):581–585. doi: 10.1016/0022-2836(86)90153-1. [DOI] [PubMed] [Google Scholar]
- Collins E. W., Jr, Edwards C. Role of Donnan equilibrium in the resting potentials in glycerol-extracted muscle. Am J Physiol. 1971 Oct;221(4):1130–1133. doi: 10.1152/ajplegacy.1971.221.4.1130. [DOI] [PubMed] [Google Scholar]
- Curtin N. A. Intracellular pH and buffer power of type 1 and 2 fibres from skeletal muscle of Xenopus laevis. Pflugers Arch. 1987 Apr;408(4):386–389. doi: 10.1007/BF00581133. [DOI] [PubMed] [Google Scholar]
- Dantzig J. A., Goldman Y. E. Suppression of muscle contraction by vanadate. Mechanical and ligand binding studies on glycerol-extracted rabbit fibers. J Gen Physiol. 1985 Sep;86(3):305–327. doi: 10.1085/jgp.86.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. K., Kerrick W. G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975 Oct;66(4):427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G. F., Bartels E. M. Donnan potential measurements in extended hexagonal polyelectrolyte gels such as muscle. Biophys J. 1982 May;38(2):195–199. doi: 10.1016/S0006-3495(82)84546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhau P., Fröhlich T., Goody R. S., Isakov M., Schirmer R. H. Synthetic inhibitors of adenylate kinases in the assays for ATPases and phosphokinases. Eur J Biochem. 1975 Sep 1;57(1):197–204. doi: 10.1111/j.1432-1033.1975.tb02291.x. [DOI] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Simmons R. M., Trentham D. R. Reaction mechanism of the magnesium ion-dependent adenosine triphosphatase of frog muscle myosin and subfragment 1. Biochem J. 1978 Apr 1;171(1):165–175. doi: 10.1042/bj1710165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Baumgarten C. M. Potential and K+ activity in skinned muscle fibers. Evidence against a simple Donnan equilibrium. Biophys J. 1984 Feb;45(2):375–382. doi: 10.1016/S0006-3495(84)84161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflugers Arch. 1981 Oct;391(4):334–337. doi: 10.1007/BF00581519. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. On the composition of the cytosol of relaxed skeletal muscle of the frog. Am J Physiol. 1988 May;254(5 Pt 1):C591–C604. doi: 10.1152/ajpcell.1988.254.5.C591. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., McCray J. A., Ranatunga K. W. Transient tension changes initiated by laser temperature jumps in rabbit psoas muscle fibres. J Physiol. 1987 Nov;392:71–95. doi: 10.1113/jphysiol.1987.sp016770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodno C. C., Taylor E. W. Inhibition of actomyosin ATPase by vanadate. Proc Natl Acad Sci U S A. 1982 Jan;79(1):21–25. doi: 10.1073/pnas.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E., Donaldson S. K., Harris C. E. Tension in skinned frog muscle fibers in solutions of varying ionic strength and neutral salt composition. J Gen Physiol. 1973 Nov;62(5):550–574. doi: 10.1085/jgp.62.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins A. B., Kurebayashi N., Baylor S. M. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys J. 1993 Aug;65(2):865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. Influence of temperature on the calcium sensitivity of the myofilaments of skinned ventricular muscle from the rabbit. J Gen Physiol. 1989 Mar;93(3):411–428. doi: 10.1085/jgp.93.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante A. A., Davies R. E. The effect of 2,4-dinitrofluorobenzene on the activity of striated muscle. J Biol Chem. 1965 Oct;240(10):3996–4001. [PubMed] [Google Scholar]
- Malan A., Wilson T. L., Reeves R. B. Intracellular pH in cold-blooded vertebrates as a function of body temperature. Respir Physiol. 1976 Oct;28(1):29–47. doi: 10.1016/0034-5687(76)90083-9. [DOI] [PubMed] [Google Scholar]
- Maughan D. W., Godt R. E. Equilibrium distribution of ions in a muscle fiber. Biophys J. 1989 Oct;56(4):717–722. doi: 10.1016/S0006-3495(89)82719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor G. R. Average electrostatic potential between the filaments in striated muscle and its relation to a simple Donnan potential. Biophys J. 1982 May;38(2):201–204. doi: 10.1016/S0006-3495(82)84547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERBEEK J. T. The Donnan equilibrium. Prog Biophys Biophys Chem. 1956;6:57–84. [PubMed] [Google Scholar]
- Padrón R., Craig R. Disorder induced in nonoverlap myosin cross-bridges by loss of adenosine triphosphate. Biophys J. 1989 Nov;56(5):927–933. doi: 10.1016/S0006-3495(89)82738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pybus J., Tregear R. T. The relationship of adenosine triphosphatase activity to tension and power output of insect flight muscle. J Physiol. 1975 May;247(1):71–89. doi: 10.1113/jphysiol.1975.sp010921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. P., Kerrick W. G. The effects of pH on Ca2+-activated force in frog skeletal muscle fibers. Pflugers Arch. 1979 May 15;380(1):41–45. doi: 10.1007/BF00582610. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Wendt I. R., Forrest Q. G. Non-uniform ion distributions and electrical potentials in sarcoplasmic regions of skeletal muscle fibres. Nature. 1981 Feb 19;289(5799):690–692. doi: 10.1038/289690a0. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Temperature-dependent calcium sensitivity changes in skinned muscle fibres of rat and toad. J Physiol. 1985 Mar;360:1–12. doi: 10.1113/jphysiol.1985.sp015600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E. D., Godt R. E. Effects of temperature and concomitant change in pH on muscle. Am J Physiol. 1990 Aug;259(2 Pt 2):R204–R209. doi: 10.1152/ajpregu.1990.259.2.R204. [DOI] [PubMed] [Google Scholar]
- Sweitzer N. K., Moss R. L. The effect of altered temperature on Ca2(+)-sensitive force in permeabilized myocardium and skeletal muscle. Evidence for force dependence of thin filament activation. J Gen Physiol. 1990 Dec;96(6):1221–1245. doi: 10.1085/jgp.96.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Methods for calibration of fluorescent calcium indicators in skeletal muscle fibers. Biophys J. 1994 Mar;66(3 Pt 1):926–928. doi: 10.1016/s0006-3495(94)80870-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiol Scand. 1988 May;133(1):83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]


