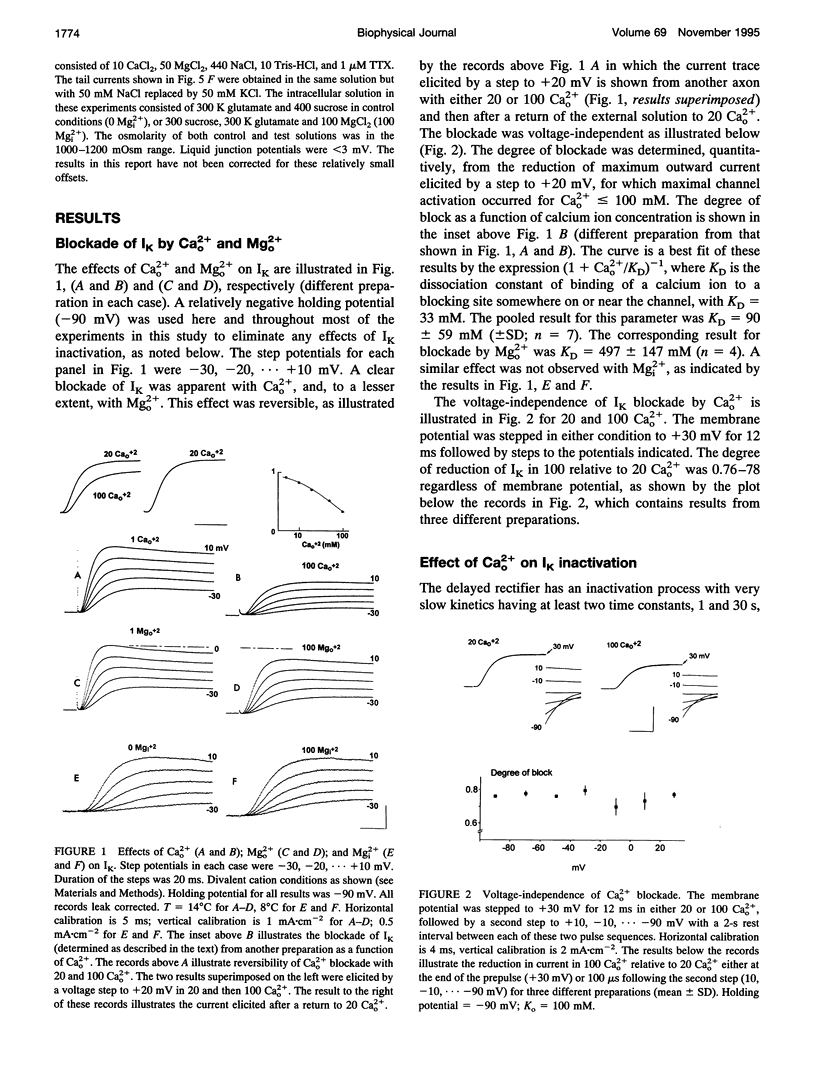
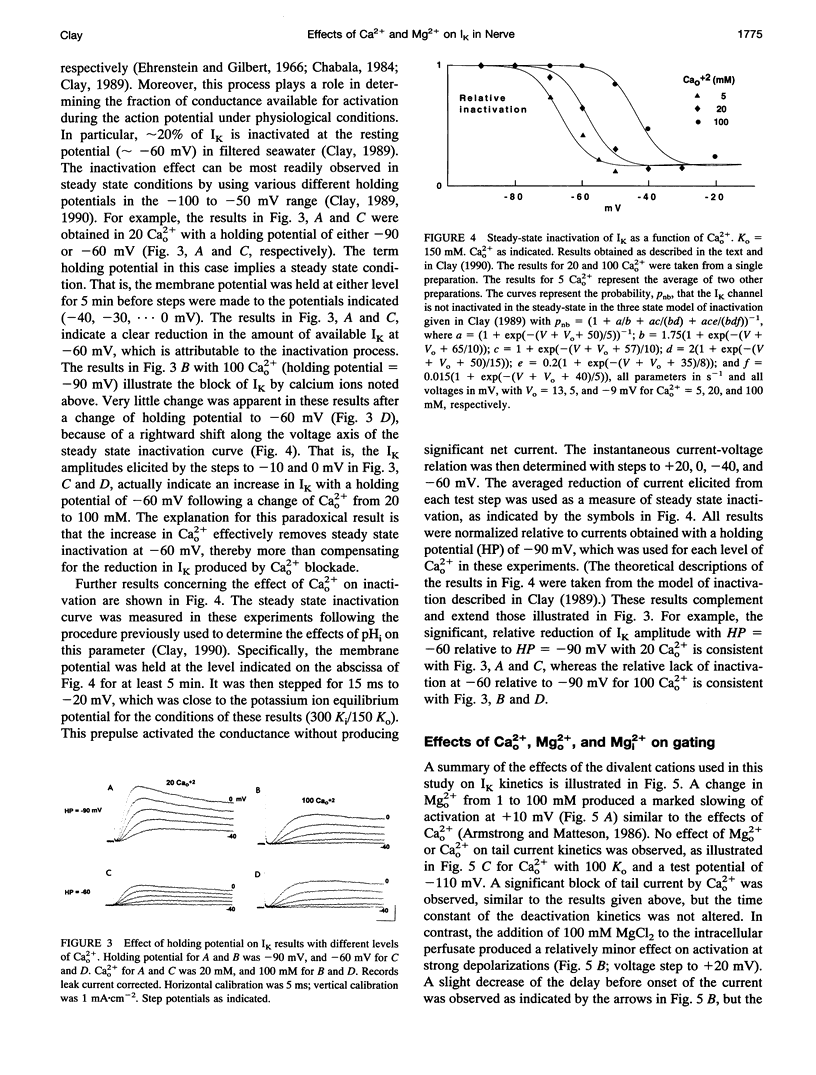
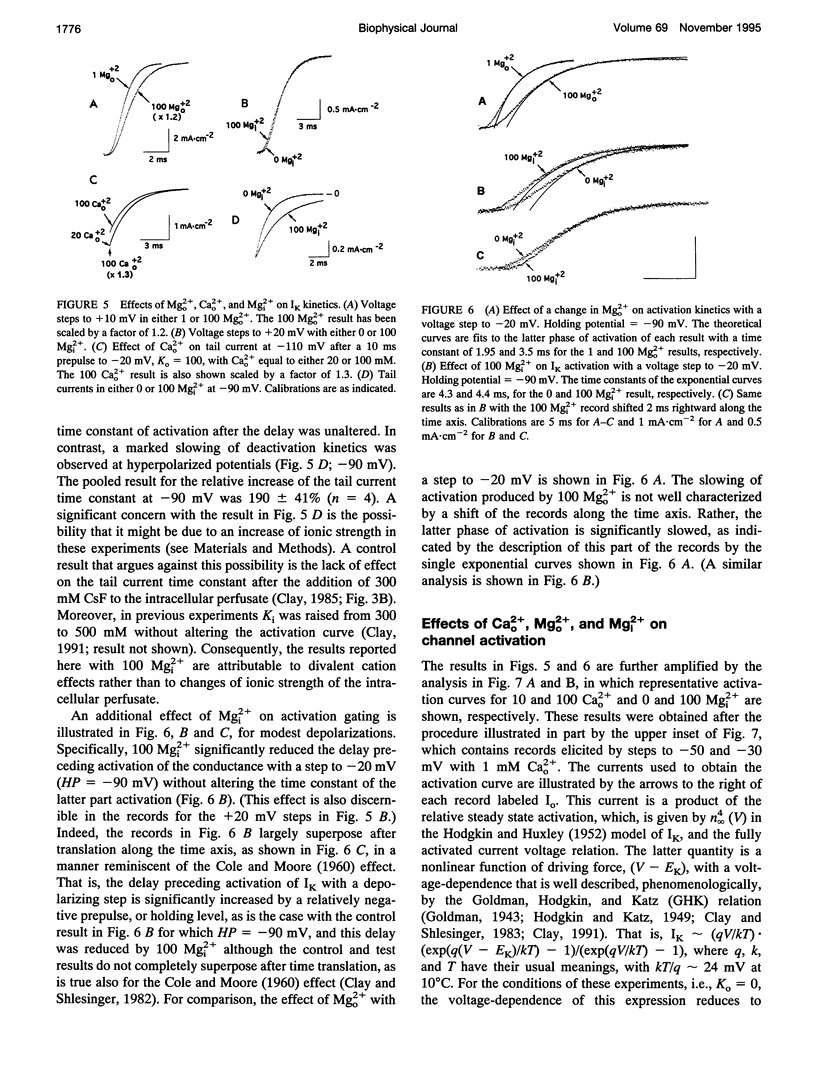
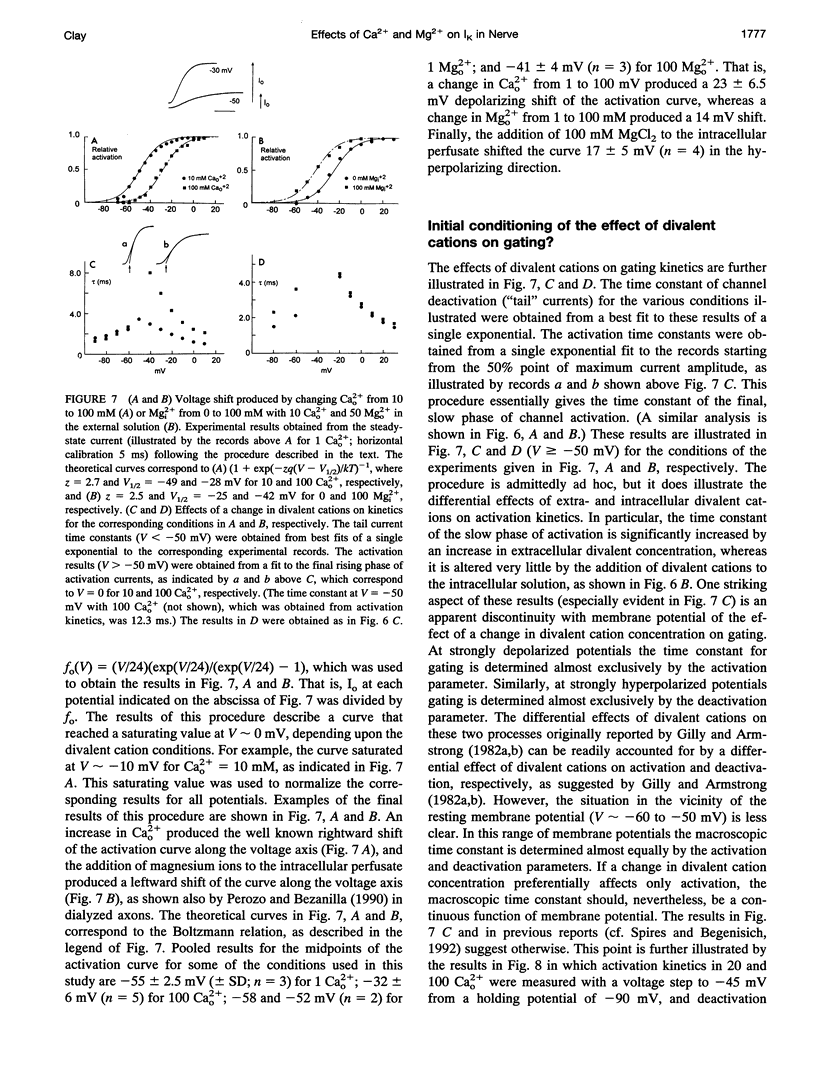
Abstract
The effects of intracellular magnesium ions and extracellular calcium and magnesium ions on the delayed rectifier potassium ion channel, IK, were investigated from intracellularly perfused squid giant axons. Cao+2 and Mgo+2 both blocked IK in a voltage-independent manner with a KD of approximately 100 and 500 mM, respectively. This effect was obscured at potentials in the vicinity of the resting potential (approximately -60 mV) by a rightward shift of the steady-state IK inactivation curve along the voltage axis. The addition of either calcium or magnesium ions to the extracellular solution also produced the well known shift of the IK activation curve along the voltage axis. Cao+2 was approximately twice as effective in this regard as Mgo+2. The IK activation kinetics were slowed by Cao+2, but deactivation kinetics were not altered, as shown previously. Similar results were obtained with Mgo+2. The addition of magnesium ions to the intracellular perfusate shifted the activation curve along the voltage axis in the negative direction (without producing block) by approximately the same among as the Mgo+2 shift of this curve in the positive direction. Moreover, Mgi+2 substantially slowed the deactivation kinetics, whereas the effects of Mgi+2 on activation kinetics at strongly depolarized potentials were relatively minor. At modest depolarizations, Mgi+2 significantly reduced the delay before IK activation. These results are essentially the mirror image of the effects on gating of extracellular divalent cations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Cota G. Modification of sodium channel gating by lanthanum. Some effects that cannot be explained by surface charge theory. J Gen Physiol. 1990 Dec;96(6):1129–1140. doi: 10.1085/jgp.96.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Matteson D. R. The role of calcium ions in the closing of K channels. J Gen Physiol. 1986 May;87(5):817–832. doi: 10.1085/jgp.87.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabala L. D. The kinetics of recovery and development of potassium channel inactivation in perfused squid (Loligo pealei) giant axons. J Physiol. 1984 Nov;356:193–220. doi: 10.1113/jphysiol.1984.sp015460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R. A paradox concerning ion permeation of the delayed rectifier potassium ion channel in squid giant axons. J Physiol. 1991 Dec;444:499–511. doi: 10.1113/jphysiol.1991.sp018890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R. IK inactivation in squid axons is shifted along the voltage axis by changes in the intracellular pH. Biophys J. 1990 Sep;58(3):797–801. doi: 10.1016/S0006-3495(90)82423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R. Potassium channel kinetics in squid axons with elevated levels of external potassium concentration. Biophys J. 1984 Feb;45(2):481–485. doi: 10.1016/S0006-3495(84)84172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., Shlesinger M. F. Delayed kinetics of squid axon potassium channels do not always superpose after time translation. Biophys J. 1982 Mar;37(3):677–680. [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., Shlesinger M. F. Effects of external cesium and rubidium on outward potassium currents in squid axons. Biophys J. 1983 Apr;42(1):43–53. doi: 10.1016/S0006-3495(83)84367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R. Slow inactivation and reactivation of the K+ channel in squid axons. A tail current analysis. Biophys J. 1989 Mar;55(3):407–414. doi: 10.1016/S0006-3495(89)82834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S., Krueger B. K. Effects of internal divalent cations on the gating of rat brain Na+ channels reconstituted in planar lipid bilayers. Pflugers Arch. 1991 Dec;419(6):559–565. doi: 10.1007/BF00370295. [DOI] [PubMed] [Google Scholar]
- Cukierman S., Krueger B. K. Modulation of sodium channel gating by external divalent cations: differential effects on opening and closing rates. Pflugers Arch. 1990 Jun;416(4):360–367. doi: 10.1007/BF00370741. [DOI] [PubMed] [Google Scholar]
- Cukierman S., Zinkand W. C., French R. J., Krueger B. K. Effects of membrane surface charge and calcium on the gating of rat brain sodium channels in planar bilayers. J Gen Physiol. 1988 Oct;92(4):431–447. doi: 10.1085/jgp.92.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Divalent cations and the activation kinetics of potassium channels in squid giant axons. J Gen Physiol. 1982 Jun;79(6):965–996. doi: 10.1085/jgp.79.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Slowing of sodium channel opening kinetics in squid axon by extracellular zinc. J Gen Physiol. 1982 Jun;79(6):935–964. doi: 10.1085/jgp.79.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., Abramson T., MacKinnon R. A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science. 1992 Nov 13;258(5085):1152–1155. doi: 10.1126/science.1279807. [DOI] [PubMed] [Google Scholar]
- Perozo E., Bezanilla F. Phosphorylation affects voltage gating of the delayed rectifier K+ channel by electrostatic interactions. Neuron. 1990 Nov;5(5):685–690. doi: 10.1016/0896-6273(90)90222-2. [DOI] [PubMed] [Google Scholar]
- Spires S., Begenisich T. Chemical properties of the divalent cation binding site on potassium channels. J Gen Physiol. 1992 Aug;100(2):181–193. doi: 10.1085/jgp.100.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Voltage-dependent calcium block of normal and tetramethrin-modified single sodium channels. Biophys J. 1984 Jan;45(1):337–344. doi: 10.1016/S0006-3495(84)84159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


