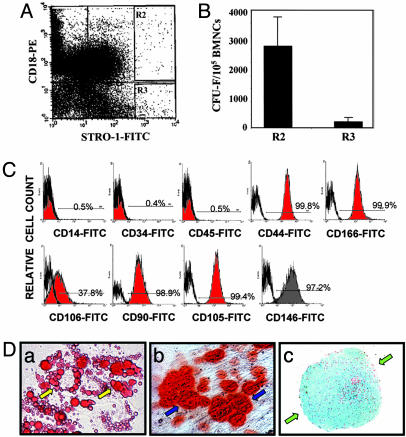
Fig. 2.
Multipotent differentiation capability of the STRO-1bright/CD18+ BMSSCs. (A and B) Enrichment of the STRO-1bright/CD18+ BMSSC population by cell sorting. Fresh human bone marrow mononuclear cells were subjected to cell sorting using both STRO-1 and CD18 as specific markers. Two distinct cell populations (R2, STRO-1bright/CD18+; and R3, STRO-1bright/CD18-) were collected (A) and subjected to CFU-F assays (B). The STRO-1bright/CD18+ cells formed much higher number of colonies than the STRO-1bright/CD18- cells. (C) Phenotypic characterization of the sorted human STRO-1bright/CD18+ BMSSCs was performed by FACS analysis using different lineage-specific mAbs (filled in red). Their corresponding isotype-matched nonimmune IgGs were used as controls (bold line). The numbers given indicate the percentages of the positive cell population. Lack of macrophage contamination of the STRO-1bright/CD18+ BMSSCs was demonstrated by the negative staining of either CD14 or CD45. (D) Differentiation potential of the STRO-1bright/CD18+ BMSSCs. The sorted STRO-1bright/CD18+ cells were cultured in vitro under adipogenic induction condition for 2 weeks (a), osteogenic induction condition for 3 weeks (b), and chondrogenic induction condition for 3 weeks (c). Oil Red O staining demonstrates the generation of lipid-laden adipocytes (yellow arrows) (a); Alizarin Red S staining shows mineral deposits (blue arrows) made by osteogenic cells (b); and Alcian blue staining of the cartilage matrix deposition (green arrows) demonstrates the chondrogenic differentiation in aggregate cultures (c). The data shown are representative of two independent experiments. (Magnification: a and b, ×400; c, ×64.)