Abstract
Fluid shear exerts anti-inflammatory and anti-apoptotic effects on endothelial cells by inducing the coordinated expression of phase 2 detoxifying and antioxidant genes. In contrast, high shear is pro-apoptotic in chondrocytes and promotes matrix degradation and cartilage destruction. We have analyzed the mechanisms regulating shear-mediated chondrocyte apoptosis by cDNA microarray technology and bioinformatics. We demonstrate that shear-induced cyclooxygenase (COX)-2 suppresses phosphatidylinositol 3-kinase (PI3-K) activity, which represses antioxidant response element (ARE)/NF-E2 related factor 2 (Nrf2)-mediated transcriptional response in human chondrocytes. The resultant decrease in antioxidant capacity of sheared chondrocytes contributes to their apoptosis. Phase 2 inducers, and to a lesser extent COX-2-selective inhibitors, negate the shear-mediated suppression of ARE-driven phase 2 activity and apoptosis. The abrogation of shear-induced COX-2 expression by PI3-K activity and/or stimulation of the Nrf2/ARE pathway suggests the existence of PI3-K/Nrf2/ARE negative feedback loops that potentially interfere with c-Jun N-terminal kinase 2 activity upstream of COX-2. Reconstructing the signaling network regulating shear-induced chondrocyte apoptosis may provide insights to optimize conditions for culturing artificial cartilage in bioreactors and for developing therapeutic strategies for arthritic disorders.
Keywords: inflammation, NAD(P)H:quinone oxidoreductase 1, NF-E2 related factor 2, phosphatidylinositol 3-kinase, sulforaphane
Fluid shear is a critical physiological stimulus that modulates intracellular signaling in a time-, magnitude-, and phenotype-dependent manner. In the vasculature, high levels of laminar shear are atheroprotective, whereas low shear oscillatory flow tends to be atherogenic. Exposure of human aortic endothelial cells to high laminar shear flow at 20 dynes (dyn)/cm2 (1 dyn = 10 μN) induces the expression of genes encoding for phase 2 detoxifying and antioxidant genes including NAD(P)H:quinone oxidoreductase 1 (NQO1), heme oxygenase (HO)-1, and γ-glutamylcysteine ligase (GCLR), which may be responsible for the resultant anti-inflammatory responses (1, 2). Moreover, laminar shear flow potently inhibits apoptosis in growth factor-starved human umbilical vein endothelial cells (HUVECs) (3). Furthermore, low intracellular glutathione (GSH) levels have been linked to mitochondrial depolarization and apoptosis in multiple cell lines (4, 5), indicating that the intracellular redox state is a key determinant of programmed cell death.
In marked contrast to the endothelium, extensive mechanical loading of cartilage producing both hydrostatic pressure and fluid shear (6) results in irreversible matrix erosion, chondrocyte apoptosis (7), and osteoarthritis. Numerous in vitro and computational studies support the view that hydrostatic pressure and low shear (<5 dyn/cm2) is chondroprotective, whereas high shear promotes matrix degradation (6, 8, 9). Indeed, high fluid shear (16 dyn/cm2) induces chondrocyte apoptosis (7), although the underlying mechanisms are unknown. We have recently shown that high shear (20 dyn/cm2) induces cyclooxygenase-2 (COX-2) expression in human chondrocytic cells through a c-Jun N-terminal kinase 2 (JNK2)-dependent pathway (10). Overexpression of COX-2 protein in articular cartilage, an earmark of arthritis (11), has been associated with inflammation and increased chondrocyte apoptosis by unknown mechanisms.
The divergent effects of fluid shear on chondrocytes and endothelial cells with regard to inflammation and apoptosis prompted us to investigate whether shear stress regulates phase 2 gene expression in chondrocytes and to elucidate the signaling pathway of shear-mediated chondrocyte apoptosis. Phase 2 enzymes protect cells against oxidative and electrophilic stress. Regulation of basal and inducible expression of cytoprotective phase 2 genes is mediated by the antioxidant response element (ARE), a cis-acting promoter element of these genes, and the cognate transcription factor, NF-E2 related factor 2 (Nrf2) (1, 12). Nrf2 is normally sequestered in the cytoplasm by binding to a repressor protein, Keap1, which is itself anchored to the actin cytoskeleton (13). When specific inducers disrupt the Nrf2/Keap1 complex, Nrf2 migrates to the nucleus where it heterodimerizes with members of the small Maf family, binds to the ARE, and enhances phase 2 gene transcription (14). Interestingly, COX-2 mediates accumulation of 15d-PGJ2 in sheared endothelial cells. This prostaglandin binds to cysteine residues of Keap1, thereby releasing Nrf2 and activating ARE-mediated transcription (2).
We show here that in human chondrocytes, shear-induced COX-2 activity suppresses phosphoinositol 3-kinase (PI3-K) activity, which leads to decreased Nrf2 expression and ARE-mediated transcription. The resultant decrease in antioxidant capacity may then modulate apoptosis. Phase 2 inducers and to a lesser extent specific inhibitors of COX-2 negate the shear-mediated repression of ARE-regulated enzyme activity and apoptosis. We provide evidence for the unexpected existence of negative feedback loops by which PI3-K activity and/or phase 2 enzyme activity repress COX-2 expression and inflammatory signaling. Elucidation of the signaling networks regulating chondrocyte apoptosis may offer novel approaches to therapy of arthritic pathology and provide insight into the design of bioreactors for culturing cartilage.
Experimental Procedures
Chemicals. Sulforaphane (SFN) and 1,2-dithiole-3-thione (D3T) were from LKT Laboratories (St. Paul, MN). NS398 and CAY10404 were from Cayman Chemical (Ann Arbor, MI).
Cell Culture and Shear Stress Exposure. Human T/C28a2 chondrocytic cells were grown (37°C in 5% CO2) in 1:1 Ham's F-12/DMEM (BioWhittaker) supplemented with 10% FBS (10, 15). Before shear exposure, T/C28a2 cells were incubated for 24 h in serum-free medium containing 1% Nutridoma-SP (Roche) (10, 15), a low-protein serum replacement that maintains chondrocyte phenotype. Primary HUVECs were cultured as described in ref. 16.
T/C28a2 cells were exposed to shear stress in media containing 1% Nutridoma by use of a parallel-plate flow chamber with a recirculating flow loop (37°C in 5% CO2) (10). HUVECs were treated similarly by circulating media supplemented with 10% FBS.
Cell Viability, NQO1 Activity, Glutathione Levels, and Prostaglandin (PG)E2 Production. Cell viability was monitored with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (17). NQO1 activity and total GSH (oxidized and reduced) levels of cell lysates were determined in 96-well microtiter plates (17). PGE2 levels were determined in media by the Prostaglandin E2 monoclonal EIA kit (Cayman Chemical).
Transient Transfection and Plasmid Constructs. T/C28a2 cells were transfected with 10 μg of plasmid and 2 μg of control by using Lipofectamine with Plus Reagent (Invitrogen). Cells were allowed to recover for 3 h, were incubated overnight in medium containing 1% Nutridoma, and were exposed to the indicated treatments. Efficiency was assessed by flow cytometry with pEGFP-N2 (BD Biosciences). pCMV-mNrf2 and pNQO1/ARE-luc constructs were provided by N. Wakabayashi (The Johns Hopkins University) (18, 19), and pBJ M·p110*·myc, pBJ M·p110·UR, and pCG p110 wt constructs were provided by A. Klippel (Atugen, Berlin) (20).
Promoter Activity Assay. T/C28a2 cells were transfected with 10 μg of pNQO1/ARE-luc and 1 μg each of pEGFP-N2 and pSV40-hRL2 (Promega) to normalize transfection efficiency. Firefly and Renilla luciferase activities were measured by using the Dual-Luciferase Reporter Assay kit (Promega).
Target Generation and Purification for Microarray Analysis. Total RNA (10 μg) isolated by using TRIzol (Invitrogen) were reverse transcribed in the presence of aminoallyl(aa)-dUTP with Super-Script II RT (Invitrogen) (10). The RNA template was hydrolyzed (0.2 M NaOH/0.1 M EDTA) at 70°C for 15 min, and unincorporated nucleotides were removed with a Microcon YM30 column (Millipore). aa-dUTP-labeled targets were dried, resuspended in 0.1 M Na2CO3 buffer (pH 9.0) (10, 21), and coupled to either NHS-Cy3 or NHS-Cy5 (Amersham Pharmacia) for 1 h at 25°C. Uncoupled label was removed with a QIAquick column (Qiagen).
Microarray Hybridization and Analysis. Cy-3- and Cy-5-labeled targets were mixed, dried, resuspended in hybridization buffer (50% formamide/10× SSC/0.2% SDS/COT-1 DNA/Poly(A)-DNA), and denatured. The targets were added to microarray slides printed with a set of 32,448 or 39,936 ESTs, allowed to hybridize at 42°C overnight, and processed as described in refs. 10 and 21. Expression ratios were derived by using TIGR Spotfinder (10, 21). Differentially expressed genes were identified by Significance Analysis of Microarrays, and analyzed with the software tmev (10).
Quantitative Real-Time PCR (qRT-PCR). qRT-PCR was used to verify DNA microarray data. Incorporation of SYBR Green into PCR products was monitored with the 7900HT detection system. Additional information is provided in Table 2, which is published as supporting information on the PNAS web site.
Intracellular Protein Staining and Western Blots. T/C28a2 cells were fixed in 1.0% formaldehyde for 10 min at 37°C, permeabilized in 90% methanol for 20 min on ice, and incubated at 25°C for 10 min in blocking buffer (0.5% BSA in PBS). Specimens were then incubated with fluorophore-conjugated monoclonal antibodies specific for COX-1 (COX-1/FITC) and COX-2 (COX-2/PE) (Cayman Chemical) or isotype controls (BD Biosciences) for 30 min, washed twice in blocking buffer, and analyzed by flow cytometry. For Western blots, total cell lysates were subjected to SDS/PAGE, transferred to a membrane, and probed with caspase-9 and β-actin antibodies (Upstate Biotechnology).
DNA Fragmentation and Mitochondrial Depolarization. For DNA fragmentation, cells were fixed in 4% paraformaldehyde for 1 h at 25°C, washed twice in PBS, and permeabilized briefly in 0.1% Triton-X100/0.1% sodium citrate on ice. Subsequently, cells were washed twice in PBS, labeled by using the In Situ Cell Death Kit (Roche), and analyzed by flow cytometry. To quantify mitochondrial membrane potential, cells were labeled by using the Mito-Probe JC-1 Kit (Molecular Probes).
Results
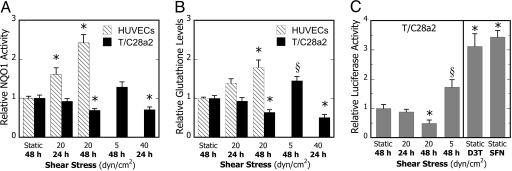
High Fluid Shear Protects Endothelial Cells but Is Proinflammatory in Chondrocytes. Recent studies indicate that laminar fluid shear stress (20 dyn/cm2) induces mRNA expression of a battery of ARE-mediated genes including those encoding for NQO1, HO-1, and GCLR in human aortic endothelial cells and may be responsible for the atheroprotective and antioxidant actions of laminar flow. Based on these observations (1), we examined the effects of fluid shear on the antioxidant capacity of T/C28a2 chondrocytic cells. Inspection of microarray data revealed that exposure of T/C28a2 cells to 20 dyn/cm2 for 1.5 or 24 h did not result in significant changes in the mRNA levels of ARE-mediated genes (Table 2), nor did it appreciably affect NQO1 activity or total GSH levels, common markers of antioxidant capacity, relative to static controls (Fig. 1 A and B). In contrast, exposure of HUVECs to 20 dyn/cm2 increased NQO1 protein activity (Fig. 1A) and GSH levels (Fig. 1B) in a time-dependent manner, in agreement with previous findings (1). We next examined the effects of longer shear duration on phase 2 response. In contrast to HUVECs, prolonged exposure (48 h) of T/C28a2 cells to 20 dyn/cm2 significantly decreased both NQO1 activity (Fig. 1A) and GSH levels (Fig. 1B), which correlates well with NQO1 and GCLR mRNA levels (Table 1).
Fig. 1.
Phenotype-specific effects of shear stress duration and intensity on phase 2 response. HUVECs and T/C28a2 chondrocytic cells were subjected to either static conditions or laminar shear flow (5, 20, or 40 dyn/cm2) for 24 or 48 h, and NQO1 specific activities (A) and total glutathione levels (per mg protein) (B) were determined. Data are relative to static controls. Bars are mean ± SEM (n = 4-7, *, P < 0.01, and §, P < 0.05, with respect to static controls). (C) ARE-driven NQO1 promoter activity in response to shear stress stimulation and phase 2 inducers. (Left) T/C28a2 cells were transfected with pNQO1/ARE-luc vector and exposed to either static conditions or laminar shear flow (5 or 20 dyn/cm2) for 24 or 48 h.(Right) To determine the efficacy of phase 2 inducers, transfected cultures were treated with solvent (0.1%), SFN (1.25 μM), or D3T (5 μM) for 24 h under static conditions. ARE-driven firefly luciferase activity was normalized to Renilla luciferase and GFP expression. Data are relative to static controls (n = 4, *, P < 0.01, and §, P < 0.05, with respect to the static control)
Table 1. Effects of phase 2 inducer D3T or transfection with PI3-K expression plasmid on mRNA transcript ratios in chondrocytes.
| Transcript ratio (shear/static)
|
|||
|---|---|---|---|
| Signaling molecules of interest | Control† | D3T‡ | Active PI3-K§ |
| Protein kinases and transcription factors | |||
| JNK2 (C-jun N-terminal kinase 2) | 7.2 ± 0.5 | 1.6 ± 0.1 | 1.3 ± 0.1 |
| PI3-K (phosphoinositol 3-kinase) | 0.3 ± 0.1 | 0.8 ± 0.2 | 3.9 ± 0.7 |
| Transcription Factor AP-1 (c-jun) | 4.0 ± 0.4 | 1.4 ± 0.1 | 1.0 ± 0.2 |
| Nrf2 (NF-E2 related factor 2) | 0.7 ± 0.1 | 1.3 ± 0.1 | 0.9 ± 0.1 |
| Catalytic and effector proteins | |||
| COX-2 (cyclooxygenase-2) | 3.5 ± 0.2 | 1.3 ± 0.2 | 1.7 ± 0.2 |
| COX-1 (cyclooxygenase-1) | 1.1 ± 0.1 | 1.3 ± 0.1 | 0.8 ± 0.2 |
| Caspase-9 precursor | 1.5 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Phase 2 and antioxidative proteins | |||
| NQO1 (NAD(P)H:quinone reductase-1) | 0.7 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 |
| HO-1 (heme oygenase-1) | 0.6 ± 0.1 | 1.2 ± 0.1 | 1.9 ± 0.2 |
| HO-2 (heme oxygenase-2) | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 |
| GST μ1 (glutathione S-transferase) | 0.5 ± 0.1 | 1.6 ± 0.1 | 1.3 ± 0.1 |
| UDP-glucuronosyltransferase | 0.7 ± 0.1 | 1.0 ± 0.1 | 1.8 ± 0.2 |
| GCLR (γ-glutamylcysteine ligase) | 0.5 ± 0.1 | 1.6 ± 0.1 | 2.0 ± 0.1 |
| Glutathione reductase | 0.4 ± 0.1 | 2.8 ± 0.1 | 2.4 ± 0.4 |
All values represent transcript ratios for sheared (20 dyn/cm2, 48 h) to paired static controls (0 dyn/cm2, 48 h) of T/C28a2 cells. Paired treatments consisted of no treatment (†); shear treated with 5 μM D3T and static treated with 0.1% DMSO (‡); and shear transfected with pBJ M·p110* (shear) and static transfected with vector only (static) (§). Data represent mean ± SD (n = 5–8).
To examine whether the reduced phase 2 response of shear-stimulated T/C28a2 cells was an artifact of prolonged shear exposure (48 h), cells were subjected to a low shear level (5 dyn/cm2) for 48 h. Interestingly, low shear resulted in a moderate increase in NQO1 activity (Fig. 1 A) and GSH levels (Fig. 1B). Moreover, shear stress exposure of 40 dyn/cm2 for 24 h resulted in a profound reduction in NQO1 activity (Fig. 1 A) and GSH levels (Fig. 1B). These findings suggest that shear stress alters the antioxidant capacity of chondrocytes in a time- and intensity-dependent manner.
High Shear Stress Suppresses ARE-Mediated Transcriptional Activity in Human Chondrocytic Cells. Prior work has established the key role of the ARE, a cis-acting DNA regulatory element of phase 2 genes, in the regulation of both their basal and inducible expression (1, 12). Microarray analysis revealed that prolonged exposure (48 h) of T/C28a2 cells to a shear level of 20 dyn/cm2 resulted in a marked reduction in Nrf2 and phase 2 transcript expression, including NQO1, HO-1, GST μ1, and GCLR (Table 1). The accuracy of the microarray transcriptional profiling was confirmed by qRT-PCR (Fig. 9, which is published as supporting information on the PNAS web site). Based on these findings, we propose that the decreased Nrf2 mRNA expression is responsible for shear-mediated suppression of phase 2 genes.
To establish involvement of the ARE in shear-mediated regulation of phase 2 genes, T/C28a2 cells were transfected with a pNQO1/ARE-luciferase plasmid and subjected to shear. Exposure of T/C28a2 cells to 20 dyn/cm2 for 48 h resulted in a substantial reduction of the ARE-driven promoter activity (Fig. 1C). In contrast, application of low shear (5 dyn/cm2) for 48 h moderately increased promoter activity (Fig. 1C Left), in agreement with enzyme activities (Fig. 1A). Having established (Fig. 10, which is published as supporting information on the PNAS web site) that T/C28a2 chondrocytic cells, like other cells, respond to chemical inducers of phase 2 activity, the functionality of the ARE element was confirmed in positive control experiments with SFN and D3T (12, 22) (Fig. 1C Right). Taken together, these data suggest that shear-mediated decrease of Nrf2 leads to repression of ARE-transcriptional activity in chondrocytes.
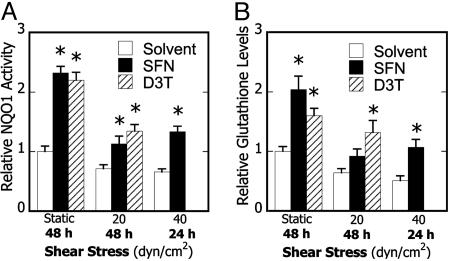
Phase 2 Inducers Restore ARE-Regulated Enzyme Activity and Reduce COX-2 Expression and PGE2 Production in Shear-Treated Chondrocytes. We next wished to reverse the reduction in antioxidant capacity and corresponding pro-inflammatory state generated in shear-treated chondrocytes. Recently, activation of the Nrf2/ARE pathway has been shown to correlate with suppression of inflammation (1, 14, 23), thus prompting the use of phase 2 inducers. Treatment of T/C28a2 cells with SFN (1.25 μM), a potent phase 2 inducer found in edible plants (22), abolished the shear-induced suppression of NQO1 activity and intracellular GSH levels (Fig. 2). Furthermore, D3T (5 μM), a specific inducer of the Nrf2/ARE pathway (12), was likewise effective in suppressing the shear-mediated reduction of phase 2 enzyme activity (Fig. 2).
Fig. 2.
Effects of phase 2 induction on shear-dependent phase 2 response in chondrocytes. Cells were treated with DMSO (0.1%), SFN (1.25 μM), or D3T (5μM) for 24 h, subjected to either static conditions or laminar shear flow (20 or 40 dyn/cm2) for 24 or 48 h in the presence of the agent, and NQO1 enzyme activity (A) and GSH levels (B) were determined. Data are relative to static controls (n = 3-9, *, P < 0.01 with respect to shear stress-paired solvent-treated controls)
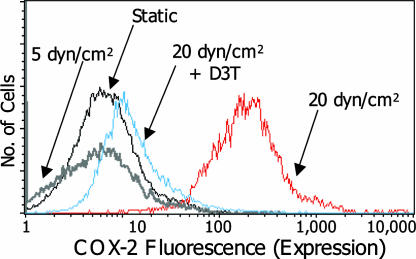
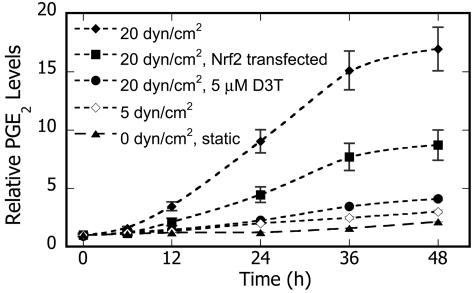
In confirmation of earlier work that application of ≥20 dyn/cm2 to chondrocytes increases COX-2 transcript levels (Table 1) (10), we observed that high, but not low, shear induced COX-2 protein expression in T/C28a2 cells (Fig. 3). Moreover, high shear sub-stantially increases COX-2-dependent PGE2 production in T/C28a2 cells in a time-dependent fashion, whereas only basal levels were detected in specimens subjected to 5 dyn/cm2 (Fig. 4). Interestingly, D3T nearly abrogated both COX-2 protein expression (Fig. 3) and PGE2 production (Fig. 4) in chondrocytes subjected to high shear. To establish involvement of Nrf2 in this process, chondrocytes were transfected with the pCMV plasmid containing murine Nrf2. This intervention substantially reduced PGE2 production (53%) in sheared T/C28a2 cells (Fig. 4), consistent with the 33% transfection efficiency of pCMV-mNrf2.
Fig. 3.
Effects of the phase 2-inducer D3T on COX-2 protein levels in shear-activated chondrocytes. Cells, treated with either solvent (0.1% DMSO) or D3T (5 μM) were subjected to either static or laminar shear (5 or 20 dyn/cm2) for 48 h in the presence of the agent. Fluorescence intensity is proportional to COX-2 expression. COX-1 expression remains unchanged (n = 3)
Fig. 4.
Effects of phase 2 induction on shear-induced COX-2-dependent prostaglandin E2 (PGE2) production in chondrocytes. Cells were treated with either solvent (0.1% DMSO) or D3T (5 μM) or transfected with pCMV-null or pCMV-mNrf2 (24 h), and then exposed to fluid shear (48 h) in the presence of the agent. PGE2 levels were determined in culture media at the indicated times. Data are relative to paired static controls at t = 0 (n = 4, transfection efficiency = 32.8 ± 4.5%)
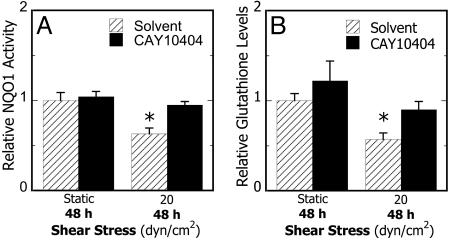
To examine the potential contribution of shear-induced COX-2 activity to the pro-inflammatory state of the T/C28a2 cells, these cells were treated with a highly selective COX-2 inhibitor, CAY10404 (6.75 μM), for 2 h before and during shear exposure (20 dyn/cm2 for 48 h). This treatment reduced the shear-induced down-regulation of NQO1 activity and intracellular GSH levels (Fig. 5). Similar results were obtained with another COX-2 inhibitor, NS398 (30 μM, data not shown). Collectively, these data provide evidence that, in chondrocytes, shear-dependent upregulation of COX-2 activity is abrogated by induction of the Nrf2-dependent phase 2 response and indicates important cross-talk between these signaling pathways.
Fig. 5.
Effects of inhibition of COX-2 activity on shear-dependent phase 2 response in chondrocytes. Cells were treated with CAY10404 (6.75 μM) or control solvent (0.1% DMSO) for 2 h, exposed to static conditions or laminar shear flow (20 dyn/cm2) for 48 h in the presence of agent, and NQO1 enzyme activities (A) and total GSH levels (B) were determined. Data obtained with NS398 (30 μM) were indistinguishable from those with CAY10404. Data are relative to static controls (n = 3-9, *, P < 0.01 with respect to static controls)
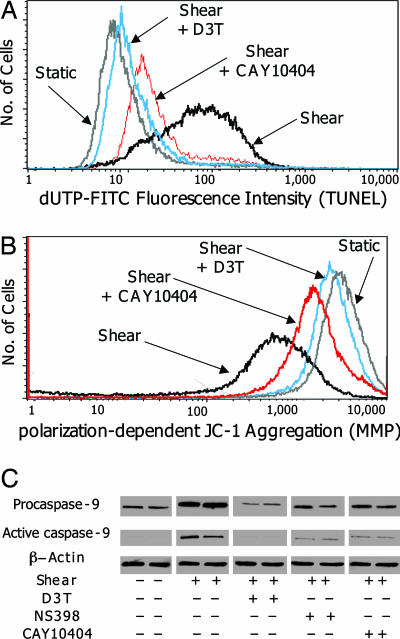
Shear-Induced Chondrocyte Apoptosis Is Suppressed by Phase 2 Inducers and COX-2 Specific Inhibitors. Microscopic inspection of shear-stimulated (20 dyn/cm2 for 48 h) T/C28a2 cells showed cell shrinkage and membrane blebbing, providing morphological evidence of apoptosis. Moreover, transcriptional profiling (Table 1) revealed increased expression of procaspase-9 mRNA, an apoptotic effector molecule activated by mitochondrial depolarization, indicating the onset of apoptosis. We then monitored the effect of shear on apoptosis by measuring DNA fragmentation (TUNEL, Fig. 6A) and mitochondrial membrane depolarization (MMP, Fig. 6B). The presence of D3T (5 μM) essentially abrogated shear-induced apoptosis, whereas treatment with the COX-2 specific inhibitors CAY10404 (6.75 μM) and NS398 (30 μM) resulted in a marked reduction in apoptosis markers (Fig. 6). Additionally, the role of caspase-9 in shear-mediated apoptosis was determined by immunoblot analysis, which revealed that high shear (20 dyn/cm2) increased the expression of both the proform (46 kDa) and active form (34 kDa) of caspase-9, whereas treatment with D3T or COX-2 selective inhibitors substantially reduced expression (Fig. 6C). Together, these results indicate that shear-mediated apoptosis proceeds by disruption of mitochondrial integrity, which is regulated by COX-2 and phase 2 enzyme activity.
Fig. 6.
Effects of phase 2 inducers and COX-2 inhibitors on shear-mediated DNA fragmentation, mitochondrial membrane permeabilization, and caspase-9 protein levels. T/C-28a2 cells were treated with the solvent (0.1% DMSO) or D3T (5 μM), for 24 h or solvent CAY10404 (6.75 μM) or NS398 (30 μM) for 2 h, and then exposed to either static or laminar flow (20 dyn/cm2) for 48 h in the presence of the agent. Cells were examined for markers of apoptosis by using DNA fragmentation (TUNEL, A), mitochondrial membrane potential (MMP, B), and caspase-9 expression (C)
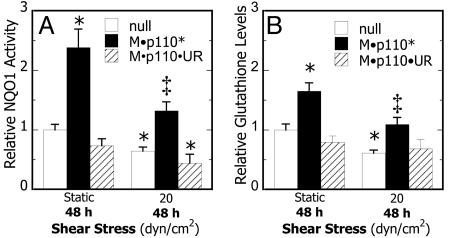
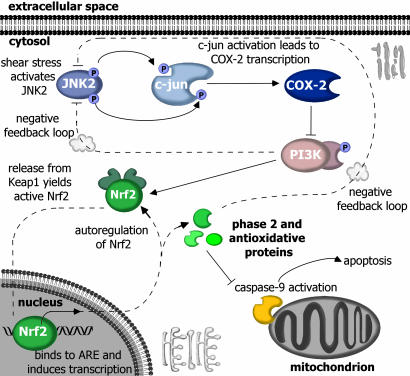
High Shear Represses PI3-K Activity That Down-Regulates Phase 2 Enzymes and Increases Apoptosis in Chondrocytes. To identify potential signaling partners involved in the down-regulation of phase 2 genes in shear-activated chondrocytes, differentially expressed genes were clustered by using support trees. Analysis established that transcriptional regulation of PI3-K (p85) paralleled that of Nrf2 and phase 2 genes, indicating that PI3-K may be involved in the shear-mediated repression of ARE-regulated transcriptional activity and the onset of apoptosis. To examine the role of PI3-K in this signaling cascade, T/C28a2 cells were transfected with a constitutively active PI3-K mutant, M·p110* and exposed to shear. This intervention prevented the shear-mediated suppression of Nrf2, phase 2 genes, and the induction of procaspase-9 (Table 1). Similarly, constitutively active PI3-K was sufficient to enhance NQO1 activity and GSH levels in static cultures and ablate their down-regulation in sheared chondrocytes (Fig. 7). Intriguingly, shear-induced COX-2 mRNA expression was markedly suppressed in chondrocytes transfected with the constitutively active form of PI3-K (Table 1) but not the wild-type construct (Table 3, which is published as supporting information on the PNAS web site). Cumulatively, these data suggest that high shear suppresses PI3-K activity, leading to the down-regulation of Nrf2 and phase 2 genes and, ultimately, to increased chondrocyte apoptosis. More importantly, our data suggest a negative feedback loop by which PI3-K activity, either directly or by activation of the Nrf2/ARE-regulated pathway, represses COX-2 expression (Fig. 8) by modulating upstream JNK2 activity (Table 1).
Fig. 7.
Effects of PI3-K activity on shear-dependent phase 2 response in chondrocytes. Cells were transfected with pBJ M·p110* (constitutively active PI3-K), pBJ M·p110·UR (Δkinase mutant), or pBJ-null vector, subjected to static conditions or laminar flow (20 dyn/cm2) for 48 h, and NQO1 enzyme activity (A) and total GSH levels (B) were determined. Data are relative to null-transfected static cultures (n = 4, *, P < 0.01 with respect to static controls; ‡, P < 0.05 with respect to null-transfected shear)
Fig. 8.
In chondrocytes, high shear flow (20 dyn/cm2) induces COX-2 expression through a JNK2/c-jun-dependent pathway, leading to PGE2 accumulation and suppression of PI3-K activity and Nrf2/ARE-mediated phase 2 response. The resultant decrease in antioxidant capacity permits disruption of mitochondrial integrity, caspase-9 activation, and apoptosis. Phase 2 inducer, PI3-K activity, and COX-2 inhibitors restore phase 2 activity. Moreover, activation of PI3-K and/or the Nrf2/ARE pathway abrogates COX-2 expression and apoptosis through negative feedback, potentially by suppressing upstream JNK2/c-jun signaling
Discussion
Chondrocytes are responsible for the production, maintenance, and repair of articular cartilage. Mechanical loading of cartilage produces hydrostatic pressure and fluid shear, both of which modulate chondrocyte function (6). Although hydrostatic pressure and low shear tend to be protective by increasing matrix production (6, 8), high fluid shear exerts pro-inflammatory effects on chondrocytes, potentially leading to matrix degradation and arthritic pathology. Additionally, recent work has linked chondrocyte apoptosis (24), aberrant COX-2 expression (11), and depleted glutathione levels (25) to the pathogenesis and progression of arthritic disorders. Therefore, from a molecular perspective, delineating the shear-regulated cross-talk among COX-2, phase 2 enzymes, and apoptosis may offer novel avenues for combating arthritic pathology.
This report examines the phenotype-specific response of chondrocytes to fluid shear by characterizing the pathway regulating shear-induced apoptosis and by identifying the roles of COX-2 and phase 2 enzymes in this process. In chondrocytes, laminar shear flow (20 dyn/cm2) induces COX-2 expression, which inhibits PI3-K activity and leads to suppression of Nrf2/ARE-mediated transcriptional activity; the decreased antioxidant capacity directly contributes to their apoptosis, characterized by cell shrinkage, membrane blebbing, DNA fragmentation, mitochondrial depolarization, and caspase-9 activation. Phase 2 inducers, and to a lesser extent COX-2 specific inhibitors, restore ARE-driven phase 2 response and prevent apoptosis, highlighting the cross-talk between COX-2 and phase 2 enzymes. The use of a constitutively active PI3-K mutant also restored Nrf2/ARE-regulated activity, thus establishing the role of PI3-K in shear-induced Nrf2 regulation. Moreover, the abrogation of shear-induced COX-2 expression by the use of phase 2 inducers or constitutively active PI3-K suggests the existence of PI3-K/Nrf2/ARE-mediated negative feedback loops, which potentially interfere with JNK2/c-jun signaling activity upstream of COX-2.
Negative feedback control is an essential regulatory mechanism by which cells maintain physiological homeostasis. We have identified a negative feedback loop in chondrocytic cells regulating the expression of pro- and anti-inflammatory molecules in response to fluid shear stress. Time-course transcriptional profiling reveals that high fluid shear activates the JNK2/c-jun/COX-2 pathway after only 1.5 h (Table 2), whereas the suppression of PI3-K activity and Nrf2/ARE-mediated transcription is not detected until 48 h (Table 1). This evidence, coupled with COX-2 inhibition experiments (Fig. 5), indicates that the JNK2/c-jun/COX-2 pathway functions upstream of PI3-K and Nrf2 (26) in the signaling cascade (Fig. 8); however, the marked inhibition of shear-induced COX-2 expression by D3T, a selective inducer of the Nrf2/ARE pathway (12), points to a negative feedback loop by which Nrf2 and/or Nrf2-regulated enzymes repress JNK2/c-jun/COX-2 expression (Table 1), potentially by interfering with JNK2/c-jun signaling or through modulation of unknown elements in the COX-2 promoter.
Functional genomics revealed that the gene expression profile of PI3-K (p85) paralleled that of Nrf2 and several phase 2 genes, indicating that decreased PI3-K activity may lead to shear-mediated suppression of ARE-regulated genes. Transfection of cells with a constitutively active PI3-K mutant, but not a wild-type construct, markedly increased Nrf2/ARE-mediated phase 2 transcription (Table 1) and enzyme activity (Fig. 7) in static and sheared chondrocytes, supporting the view that PI3-K is a key regulator of Nrf2-mediated transcription (27, 28). These findings, coupled with time-course transcriptional profiling, suggest that PI3-K may mediate communication between COX-2 and the phase 2 response. The existence of such cross-talk is supported by the findings that COX-2 expression regulates PI3-K activity in UVB-irradiated human lung adenocarcinoma cells (29). Interestingly, constitutively active PI3-K also attenuated shear-induced JNK2/c-jun/COX-2 up-regulation (Table 1), potentially through interaction with JNK2 (30); however, because PI3-K activity also stimulates ARE-driven gene expression, it is unclear whether PI3-K affects COX-2 expression directly, through expression of phase 2 enzymes (Fig. 8) or through other mediators.
In addition to its role in detoxification and cellular defense, Nrf2 has been implicated in the regulation of inflammation in macrophages (14) and endothelial cells (1, 23). The ability of phase 2 inducers to abrogate shear-induced COX-2 expression and PGE2 production in chondrocytes presents a previously undescribed approach for the prevention of cartilage inflammation. This view is supported by similar work in IFN-γ-stimulated macrophages showing that phase 2 inducers can suppress COX-2 and iNOS transcription, and that the potency of NQO1 induction and COX-2 suppression is highly correlated (14). Moreover, recent evidence that the shear-induced up-regulation of phase 2 genes in human aortic endothelial cells is attenuated by COX-2-specific inhibitors (2) may contribute to their cardiovascular side effects (31). Whereas COX-2 inhibitors reduce inflammation in cartilage, their presence in the vasculature prevents the accumulation of COX-2-derived 15d-PGJ2, which is associated with the atheroprotective nature of laminar flow. Consequently, phase 2 inducers represent an attractive and safe alternative to COX-2 inhibitors based on their anti-inflammatory potential and their highly beneficial antioxidative properties.
These findings highlight the existing cross-talk among COX-2 expression, reduced antioxidant capacity, and increased apoptosis in sheared chondrocytic cells, all earmarks of arthritis. Reconstructing the biochemical pathways regulating cartilage inflammation and chondrocyte apoptosis in response to high shear stress may identify potential therapeutic targets for controlling arthritic pathogenesis and/or progression and may be useful in the design of bioreactors for cartilage culture.
Supplementary Material
Acknowledgments
We thank J. Quackenbush for insightful discussions, N. Wakabayashi for the pNQO1/ARE-luc and pCMV-mNrf2 constructs, and A. Klippel for the PI3-K mutants. This work was supported, in part, by a DuPont Young Professor Award (to K.K.), a National Science Foundation Graduate Research Fellowship, and an Achievement Rewards for College Students Fellowship (to Z.R.H.). Establishment of the cell line was supported by National Institutes of Health Grant R01-AG22021 (to M.B.G.).
Author contributions: Z.R.H., N.H.L., X.G., P.T., T.W.K., and K.K. designed research; Z.R.H. performed research; N.H.L., M.B.G., P.T., and T.W.K. contributed new reagents/analytic tools; Z.R.H., X.G., P.T., and K.K. analyzed data; and Z.R.H., P.T., T.W.K., and K.K. wrote the paper.
Abbreviations: ARE, antioxidant response element; COX, cyclooxygenase; D3T, 1,2-dithiole-3-thione; dyn, dyne; GCLR, γ-glutamylcysteine ligase regulatory unit; GSH, glutathione; HO, heme oxygenase; HUVECs, human umbilical vein endothelial cells; JNK2, c-jun N-terminal kinase 2; Keap1, Kelch-like ECH-associated protein 1; NQO1, NAD(P)H:quinone oxidoreductase 1; Nrf2, NF-E2 related factor 2; PG, prostaglandin; PI3-K, phosphoinositol 3-kinase; SFN, sulforaphane.
References
- 1.Chen, X. L., Varner, S. E., Rao, A. S., Grey, J. Y., Thomas, S., Cook, C. K., Wasserman, M. A., Medford, R. M., Jaiswal, A. K. & Kunsch, C. (2003) J. Biol. Chem. 278, 703-711. [DOI] [PubMed] [Google Scholar]
- 2.Hosoya, T., Maruyama, A., Kang, M. I., Kawatani, Y., Shibata, T., Uchida, K., Itoh, K. & Yamamoto, M. (2005) J. Biol. Chem. 280, 27244-27250. [DOI] [PubMed] [Google Scholar]
- 3.Dimmeler, S., Haendeler, J., Rippmann, V., Nehls, M. & Zeiher, A. M. (1996) FEBS Lett. 399, 71-74. [DOI] [PubMed] [Google Scholar]
- 4.Jang, J. H. & Surh, Y. J. (2003) Biochem. Pharmacol. 66, 1371-1379. [DOI] [PubMed] [Google Scholar]
- 5.Voehringer, D. W., Hirschberg, D. L., Xiao, J., Lu, Q., Roederer, M., Lock, C. B., Steinman, L. & Herzenberg, L. A. (2000) Proc. Natl. Acad. Sci. USA 97, 2680-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter, D. R., Beaupre, G. S., Wong, M., Smith, R. L., Andriacchi, T. P., Schurman, D. J. & Smith, R. L. (2004) Clin. Orthop. Relat. Res. 427, Suppl., S69-S77. [DOI] [PubMed] [Google Scholar]
- 7.Lee, M. S., Trindade, M. C. D., Ikenoue, T., Goodman, S. B., Schurman, D. J. & Smith, R. L. (2003) J. Cell. Biochem. 90, 80-86. [DOI] [PubMed] [Google Scholar]
- 8.Smith, R. L., Carter, D. R., Schurman, D. J. & Smith, R. L. (2004) Clin. Orthop. Relat. Res. 427, Suppl., S89-S95. [PubMed] [Google Scholar]
- 9.Yokota, H., Goldring, M. B. & Sun, H. B. (2003) J. Biol. Chem. 278, 47275-47280. [DOI] [PubMed] [Google Scholar]
- 10.Abulencia, J. P., Gaspard, R., Healy, Z. R., Gaarde, W. A., Quackenbush, J. & Konstantopoulos, K. (2003) J. Biol. Chem. 278, 28388-28394. [DOI] [PubMed] [Google Scholar]
- 11.Amin, A. R., Attur, M., Patel, R. N., Thakker, G. D., Marshall, P. J., Rediske, J., Stuchin, S. A., Patel, I. R. & Abramson, S. B. (1997) J. Clin. Invest. 99, 1231-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwak, M. K., Wakabayashi, N., Itoh, K., Motohashi, H., Yamamoto, M. & Kensler, T. W. (2003) J. Biol. Chem. 278, 8135-8145. [DOI] [PubMed] [Google Scholar]
- 13.Kang, M.-I., Kobayashi, A., Wakabayashi, N., Kim, S.-G. & Yamamoto, M. (2004) Proc. Natl. Acad. Sci. USA 101, 2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinkova-Kostova, A. T., Liby, K. T., Stephenson, K. K., Holtzclaw, W. D., Gao, X. Q., Suh, N., Williams, C., Risingsong, R., Honda, T., Gribble, G. W., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 4584-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldring, M. B. (2004) Methods Mol. Med. 100, 37-52. [DOI] [PubMed] [Google Scholar]
- 16.Konstantopoulos, K., Kukreti, S., Smith, C. W. & McIntire, L. V. (1997) J. Leukoc. Biol. 61, 179-187. [DOI] [PubMed] [Google Scholar]
- 17.Gao, X., Dinkova-Kostova, A. T. & Talalay, P. (2001) Proc. Natl. Acad. Sci. USA 98, 15221-15226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakabayashi, N., Dinkova-Kostova, A. T., Holtzclaw, W. D., Kang, M.-I., Kobayashi, A., Yamamoto, M., Kensler, T. W. & Talalay, P. (2004) Proc. Natl. Acad. Sci. USA 101, 2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igarashi, K., Kataoka, K., Itoh, K., Hayashi, N., Nishizawa, M. & Yamamoto, M. (1994) Nature 367, 568-572. [DOI] [PubMed] [Google Scholar]
- 20.Hu, Q., Klippel, A., Muslin, A. J., Fantl, W. J. & Williams, L. T. (1995) Science 268, 100-102. [DOI] [PubMed] [Google Scholar]
- 21.Hegde, P., Qi, R., Abernathy, K., Gay, C., Dharap, S., Gaspard, R., Hughes, J. E., Snesrud, E., Lee, N. & Quackenbush, J. (2000) Biotechniques 29, 548-556. [DOI] [PubMed] [Google Scholar]
- 22.Fahey, J. W., Haristoy, X., Dolan, P. M., Kensler, T. W., Scholtus, I., Stephenson, K. K., Talalay, P. & Lozniewski, A. (2002) Proc. Natl. Acad. Sci. USA 99, 7610-7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, X. L. & Kunsch, C. (2004) Curr. Pharm. Des. 10, 879-891. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto, S., Ochs, R. L., Komiya, S. & Lotz, M. (1998) Arthritis Rheum. 41, 1632-1638. [DOI] [PubMed] [Google Scholar]
- 25.Hassan, M. Q., Hadi, R. A., Al-Rawi, Z. S., Padron, V. A. & Stohs, S. J. (2001) J. Appl. Toxicol. 21, 69-73. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki, M., Ishii, Y., Itoh, K., Iizuka, T., Morishima, Y., Kimura, T., Kiwamoto, T., Matsuno, Y., Hegab, A. E., Nomura, A., et al. (2005) Am. J. Respir. Crit. Care Med. 171, 1260-1266. [DOI] [PubMed] [Google Scholar]
- 27.Lee, J. M., Hanson, J. M., Chu, W. A. & Johnson, J. A. (2001) J. Biol. Chem. 276, 20011-20016. [DOI] [PubMed] [Google Scholar]
- 28.Nakaso, K., Yano, H., Fukuhara, Y., Takeshima, T., Wada-Isoe, K. & Nakashima, K. (2003) FEBS Lett. 546, 181-184. [DOI] [PubMed] [Google Scholar]
- 29.Lin, M. T., Lee, R. C., Yang, P. C., Ho, F. M. & Kuo, M. L. (2001) J. Biol. Chem. 276, 48997-49002. [DOI] [PubMed] [Google Scholar]
- 30.Aikin, R., Maysinger, D. & Rosenberg, L. (2004) Endocrinology 145, 4522-4531. [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee, D. (2002) Biochem. Pharmacol. 63, 817-821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.