Abstract
Purpose
This study aims to examine the relationship between early fetal hemoglobin (HbF) decline, as a marker of oxidative stress, and the risk of bronchopulmonary dysplasia (BPD) in very low birth weight (VLBW) infants, with a focus on identifying critical risk thresholds.
Methods
A prospective cohort study was conducted on VLBW infants admitted to the First Hospital of Jilin University. HbF decline was assessed by measuring absolute reduction and rate of decline within the first 7 days of life. The primary outcomes were mortality and/or development of BPD.
Results
A total of 294 infants were included in the study, of whom 5 (1.70%) died, and 142 (48.3%) developed BPD. A nonlinear relationship between HbF decline in the first 7 days and the primary outcomes was observed. Thresholds of 3 g/L for absolute HbF decline and 0.05 for the rate of decline were identified using a two-piecewise linear regression model. Infants were classified into low and high HbF decline groups based on these thresholds. Multivariate logistic regression showed a significantly higher risk of BPD in the high HbF decline group (OR = 2.77, 95% CI 1.52–5.02, P < 0.001) Subgroup analyses largely confirmed these findings.
Conclusions
A nonlinear correlation was identified between HbF decline in the first 7 days and the risk of mortality and/or BPD in VLBW infants. An absolute HbF decline > 3 g/L or a rate > 0.05 was significantly associated with increased risk, offering a basis for BPD risk prediction.
Keywords: Bronchopulmonary dysplasia, Fetal hemoglobin, Very low birth weight infants, Smooth curve fitting
Introduction
Bronchopulmonary Dysplasia (BPD) is among the most common and severe complications in preterm infants and is a significant contributor to reduced long-term quality of life in these patients. Chronic ischemia, hypoxia, and oxidative stress are critical pathogenic factors in the development of this condition [1, 2]. The transition from the intrauterine to the extrauterine environment in preterm infants often involves a shift from a relatively hypoxic state to a hyperoxic one [3]. Early postnatal exposure to higher oxygen levels generates a substantial amount of reactive oxygen species (ROS), overwhelming the limited antioxidant defenses of preterm infants. Oxidative stress induced by ROS can damage various cellular components, thereby increasing the risk of BPD in this vulnerable population [4].
Fetal Hemoglobin (HbF) is a key component of hemoglobin in preterm infants, and it is the predominant type of hemoglobin in preterm infants during the early postnatal period [5, 6]. Compared with adult hemoglobin (HbA), HbF is characterized by its high oxygen affinity, antioxidant capacity, and structural stability [7]. However, due to various factors such as hemolysis and blood transfusions, the proportion of HbF in preterm infants rapidly decreases after birth. This results in a rightward shift of the oxygen dissociation curve [8], which, under the same inspired oxygen concentration, leads to an increased proportion of dissolved oxygen in the plasma and a greater accumulation of ROS in lung tissue.
Recent clinical studies have found that early postnatal changes in HbF levels are associated with the development of BPD [9]. For instance, Hellström et al. observed a negative correlation between the average HbF level during the first week after birth and the risk of BPD in a cohort of 452 extremely preterm infants. Given the lack of established reference ranges for HbF levels in preterm infants, it is therefore particularly valuable to investigate whether a decline in HbF levels during the first week of life is associated with BPD, and, if so, to determine whether a critical threshold exists for this association.
This study employs a prospective cohort design to investigate the relationship between the extent of postnatal HbF decline and the risk of mortality and/or BPD in VLBW infants. Furthermore, it aims to establish the HbF threshold that significantly increases the risk of developing BPD in this population. The findings will offer crucial insights for early identification and intervention in preterm infants at high risk for BPD.
Methods
Study design
This study is a prospective cohort investigation involving VLBW infants admitted to the Neonatology Department of the First Hospital of Jilin University within 24 h of birth between April 2023 and April 2024. The research has received ethical approval from the Ethics Committee of the First Hospital of Jilin University (Ethics Approval No. 23K005-001) in accordance with the Declaration of Helsinki and is registered with ClinicalTrials.gov (Registration No. NCT05788562, registered on April 15, 2023). Informed consent was obtained from the parents or guardians of all participating infants.
Population
The study included VLBW infants with a birth weight of less than 1500 g who were admitted to the Neonatology Department of the First Hospital of Jilin University within 24 h of birth. Exclusion Criteria includes: Infants with congenital anomalies, complex congenital heart disease, or organic diseases of the liver, kidneys, or other organs; Infants for whom treatment was discontinued within the first 7 days of life; Preterm infants with incomplete critical data (e.g., HbF levels, primary outcome measures such as mortality and BPD).
Exposure
The exposure factors in this study are the absolute decrease in HbF levels and the rate of HbF decline during the first week of life. HbF levels were measured through blood gas analysis based on the principle of multi-wavelength spectrophotometric analysis (co-oximetry) [10, 11], with the absolute decrease in HbF calculated as the difference between the HbF levels on day 1 and day 7. The rate of HbF decline was determined as the ratio of this absolute decrease to the HbF level on day 1. If multiple blood gas analyses were conducted on a single day, the average HbF level for that day was used.
Primary outcome measures
The primary outcome measures were mortality and/or BPD. BPD was defined according to the 2019 National Institute of Child Health and Human Development Neonatal Research Network (NRN) criteria [12], as the requirement for supplemental oxygen and/or noninvasive or invasive respiratory support at 36 weeks’ corrected gestational age.
Variables
Baseline characteristics included sex, gestational age, birth weight, mode of delivery, presence of meconium-stained amniotic fluid, 1-minute and 5-minute Apgar scores, presence of premature rupture of membranes, maternal hypertension or diabetes, and the use of antenatal corticosteroids. Additional data collected encompassed the need for invasive mechanical ventilation within the first 7 days of life, the volume of iatrogenic blood loss within the same period, and whether a blood transfusion was administered within the first 7 days.
Sample size and power calculations
The statistical power of this study was estimated with a significance level (α) of 0.05 and an anticipated effect size defined by the expected risk ratio, informed by the event rates observed in comparable populations reported in the literature. The power analysis was performed using PASS 11 software.
Statistical analysis
All statistical analyses were performed using R software (vision 4.3.1). Continuous variables with a normal distribution were expressed as mean ± standard deviation and compared between groups using independent samples t-tests. For continuous variables that did not follow a normal distribution, data were presented as median and interquartile range (IQR) and compared using the Mann-Whitney U test. Categorical variables were presented as frequencies and percentages (n, %) and analyzed using the chi-square test. A smoothing spline technique was applied to investigate potential nonlinear relationships between exposure factors and outcome measures [13]. Segmented regression was used to fit separate linear segments for each interval, and the log-likelihood ratio test was employed to determine the presence of a threshold effect by comparing a simple linear model with a segmented regression model. Based on the identified threshold, groups were stratified, and univariate analysis was conducted. After adjusting for confounders, multivariate logistic regression analysis was performed. Subgroup analyses were carried out based on gestational age, birth weight, maternal hypertension, and the administration of a blood transfusion within the first 7 days. Multivariate logistic regression was further utilized to validate the association between HbF levels and complications in preterm infants across different subpopulations.
Results
Baseline characteristics of participants
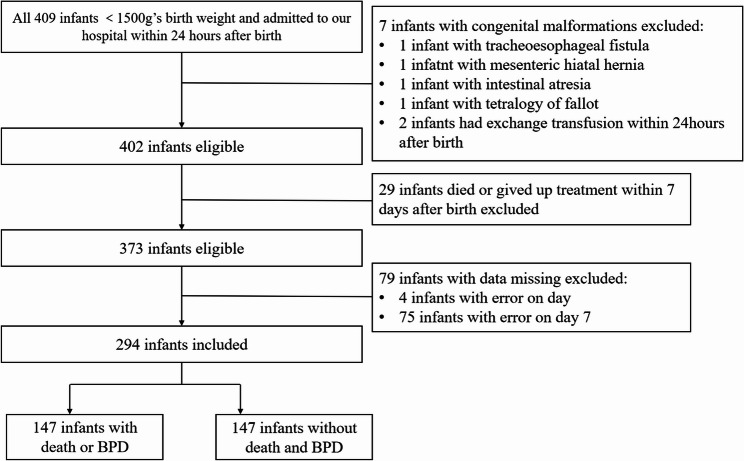
A total of 409 VLBW infants were admitted to our institution within 24 h of birth. Exclusions were made for cases with significant congenital anomalies, including one instance each of tracheoesophageal fistula, omphalocele, and intestinal atresia, as well as two cases of tetralogy of Fallot. Additionally, one infant received an exchange transfusion within 24 h, and one case of Down syndrome was identified as a genetic metabolic disorder. Twenty-six infants had treatment withdrawn within the first 7 days, and 4 infants had missing data for HbF on day 1, with 75 missing HbF data on day 7. Ultimately, 294 infants were included in the analysis, with 147 meeting criteria for either death or BPD, and 147 not reaching the outcome measures. The screening process is depicted in Fig. 1.
Fig. 1.
Flow chart of study population
A total of 294 infants were included in the study. The baseline characteristics are detailed in Table 1. The median gestational age was 29 weeks (IQR: 27, 30), and the median birth weight was 1170 g (IQR: 960, 1350). The median iatrogenic blood loss within the first 7 days was 9.5 ml (IQR: 7.5, 11.5). Antenatal corticosteroids were administered to 211 infants (71.77%), and 62 infants (21.09%) received a blood transfusion within the first 7 days. Among the 294 infants, 147 (50.0%) experienced the primary outcome: 5 (1.70%) died and 142 (48.3%) developed BPD.
Table 1.
Baseline characteristics of participants
| Characteristics | n = 294 |
|---|---|
| Male (n, %) | 168 (57.14%) |
| Birth weight (g) (median, IQR) | 1170 (960, 1350) |
| Gestational age (weeks) (median, IQR) | 29 (27, 30) |
| Cesarean section (n, %) | 211 (69.87%) |
| 1-min low Apgar score (≤ 7) (n, %) | 237(80.61%) |
| 5-min low Apgar score (≤ 7) (n, %) | 113 (38.4%) |
| Multiple birth (n, %) | 99 (33.67%) |
| Small for gestational age (n, %) | 49 (16.67%) |
| Maternal hypertension (n, %) | 110 (37.41%) |
| Maternal diabetes (n, %) | 88 (29.93%) |
| Rupture of membrane (n, %) | 80 (27.21%) |
| Antenatal steroids use (n, %) | 211 (71.77%) |
| Iatrogenic blood loss within 7 days (ml) (median, IQR) | 9.5 (7.5, 11.5) |
| Blood transfusion within 7 days (n, %) | 62 (21.09%) |
Relationship between early postnatal decline in HbF and BPD
We utilized generalized additive models to visually assess the relationship between continuous covariates (HbF decrease values and decrease rates) and the risk of mortality and/or BPD (Fig. 2A and B). The smoothing spline fitting indicated a nonlinear relationship between HbF decrease values or decrease rates and the risk of mortality and/or BPD (Fig. 2). Further analysis identified a threshold for HbF decrease value at 3 g/L, where a decrease value greater than 3 g/L was positively associated with the risk of mortality and/or BPD (OR = 1.152, 95% CI 1.076–1.234, P < 0.001). The threshold for HbF decrease rate was found to be 0.05; a decrease rate greater than 0.05 was positively correlated with the risk of complications (OR = 510375.939, 95% CI 776.411–infinity, P < 0.001). In addition, a decrease value greater than 26 g/L or a decrease rate greater than 0.35, the upward trend was not obvious, the difference was not statistical significant (P = 0.126, P = 0.054)(Table 2).
Fig. 2.
Dose-Response Curves of HbF decline value or rate and the mortality and/or BPD in VLBW. (A) Association between the decrease in HbF levels and the mortality and/or BPD, with a threshold of 3. (B) Association between the rate of HbF decline and the mortality and/or BPD, with a threshold of 0.05. (Adjusted by gestational age, maternal hypertension, iatrogenic blood loss within the first 7 days, and blood transfusion within the first 7 days.)
Table 2.
Threshold effects of HbF decline value or rate on the risk of mortality and/or BPD in VLBW infants (Adjusted by gestational age, maternal hypertension, iatrogenic blood loss within the first 7 days, and blood transfusion within the first 7 days.)
| Chracteristics | HbF decline value | HbF decline rate |
|---|---|---|
| β (95%CI), P value | β (95%CI), P value | |
| Model I | ||
| One line effect | 1.094 (1.048,1.142), 0.003 | 2086.519 (56.375,77224.800), < 0.001 |
| Model II | ||
| Breakpoint(K) | 3 | 0.05 |
| < K effect 1 | 0.995 (0.904,1.096) 0.924 | 1.619 (0.002,1720.279) 0.892 |
| >K effect 2 | 1.152 (1.076, 1.234) < 0.001 | 510375.939 (776.411, inf.), < 0.001 |
| Effect 2 − 1 | 1.158 (1.009, 1.328) 0.037 | 315325.235 (4.820, inf.) 0.025 |
| Model fit value at K | −0.657 (−1.047, −0.266) | −0.555 (−0.935, −0.174) |
| LRT test | 0.034 | 0.021 |
| Model I | ||
| One line effect | 1.087(1.043,1.133), <0.001 | 1103.17 (35.54,34239.89), < 0.001 |
| Model II | ||
| Breakpoint(K) | 26 | 0.35 |
| < K effect 1 | 1.103 (1.054,1.155) < 0.001 | 4849.532 (111.134,211618.270) < 0.001 |
| >K effect 2 | 0.964 (0.836, 1.113) 0.620 | 0.001 (0.00, 367.375.), 0.283 |
| Effect 2 − 1 | 0.874 (0.744, 1.027) 0.101 | 0.000 (0.000, 0.343.) 0.036 |
| Model fit value at K | 1.878 (1.076, 2.680) | 2.299 (1.385, 3.213) |
| LRT test | 0.126 | 0.054 |
(Adjusted by gestational age, maternal hypertension, iatrogenic blood loss within the first 7 days, and blood transfusion within the first 7 days.)
Risk of mortality and/or BPD among high and low HbF decline groups
Infants were categorized based on an HbF decline rate threshold of 0.05. Specifically, 130 infants with a decline rate ≤ 0.05 were classified as the low HbF decline group, while 164 infants with a decline rate > 0.05 were classified as the high HbF decline group. The baseline characteristics of both groups are shown in Table 3.
Table 3.
Baseline characteristics of high and low HbF decline groups
| Basic information | Low HbF decline group n = 130 | High HbF decline group n = 164 | X2/Z | P value |
|---|---|---|---|---|
| Male (n) | 74 (56.9%) | 94 (57.3%) | 0.005 | 0.946 |
| Birth weight (g) | 1285 (1060,1400) | 1100 (890,1285) | −4.810 | <0.001 |
| Gestational age (w) | 29 (28,31) | 28 (27,30) | −3.572 | <0.001 |
| Cesarean section (n) | 92 (70.7%) | 113 (68.9%) | 0.120 | 0.729 |
| 1-min low Apgar score (n) | 101 (77.7%) | 135 (82.3%) | 1.215 | 0.270 |
| 5-min low Apgar score (n) | 45 (34.6%) | 65 (39.6%) | 0.854 | 0.355 |
| Twins (n) | 45 (34.6%) | 54 (32.9%) | 0.093 | 0.761 |
| SGA (n) | 20 (15.4%) | 29 (17.7%) | 0.276 | 0.599 |
| Maternal hypertension (n) | 43 (33.0%) | 67 (40.9%) | 1.873 | 0.171 |
| Maternal diabetes (n) | 34 (26.2%) | 54 (32.9%) | 1.586 | 0.208 |
| Rupture of membrane (n) | 39 (30.0%) | 41 (25.0%) | 0.915 | 0.339 |
| Antenatal steroids use (n) | 98 (75.4%) | 113 (68.9%) | 1.504 | 0.220 |
| Iatrogenic blood loss within 7 days(ml) | 8.5 (7.0, 10.5) | 10.5 (8.0, 14.0) | −4.390 | <0.001 |
| Blood transfusion within 7 days (n) | 1 (0.01%) | 33 (20.1%) | 26.556 | <0.001 |
| Death or BPD (n) | 43 (33.1%) | 104 (63.4%) | 26.697 | <0.001 |
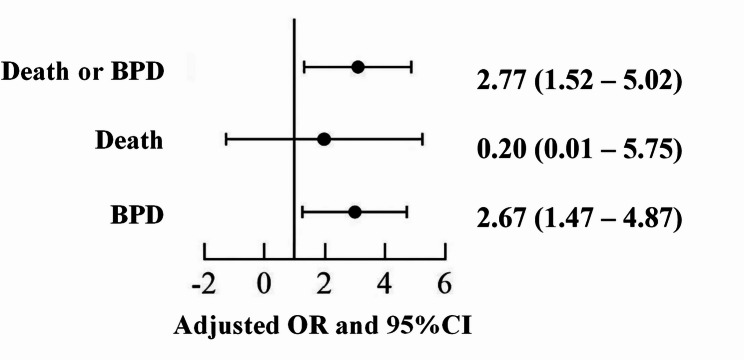
A multivariate logistic regression analysis was conducted to adjust for confounding variables, including maternal infection, antenatal steroids use during gestation, gestational age, gender, multiple births, small for gestational age status, maternal hypertension, iatrogenic blood loss within the first 7 days, and blood transfusion within the first 7 days. Infants in the high HbF decline rate group exhibited a significantly increased risk of mortality and/or BPD (OR = 2.77, 95% CI 1.52–5.02, P = 0.001) (see Fig. 3).
Fig. 3.
Multiple logistic regression: risk of mortality and/or BPD among high and low HbF decline groups (Adjusted by maternal infection, antenatal steroids use during gestation, gestational age, gender, multiple births, small for gestational age status, maternal hypertension, iatrogenic blood loss within the first 7 days, and blood transfusion within the first 7 days.)
Subgroups analyses of high and low HbF decline groups
To further evaluate the association between HbF decline and BPD in preterm infants, we conducted a subgroup analysis stratified by gestational age, birth weight, maternal hypertension, and blood transfusion within 7 days. In subgroups with a gestational age of ≥ 30 weeks, non-maternal hypertension and those who received a blood transfusion within 7 days, there was a noted trend towards a decreased risk of mortality or BPD. In all other subgroups, infants with high HbF decline consistently exhibited a heightened risk of mortality or BPD (see Fig. 4).
Fig. 4.
Risk of mortality and/or BPD among high and low HbF decline groups in subgroups (Adjusted by maternal infection, antenatal steroids use during gestation, gestational age, gender, multiple births, small for gestational age status, maternal hypertension, iatrogenic blood loss within the first 7 days, and blood transfusion within the first 7 days.)
Discussion
This prospective cohort study of 294 VLBW infants revealed that, during the first week of life, both the magnitude of HbF decline and the rate of HbF decline exhibit a non-linear association with mortality or BPD. Specifically, an HbF decline value greater than 3 g/Lor a decline rate exceeding 0.05 are associated with an increased risk of BPD in VLBW infants.
The finding in this study that high HbF decline is associated with an increased risk of BPD aligns with the results reported by Hellström et al. [9]. Hellström and colleagues investigated preterm infants with a gestational age of less than 30 weeks and demonstrated that a 10% higher of HbF levels during the first week of life was associated with a reduced incidence of BPD. The elevated risk of BPD observed in the high HbF decline group may be attributable to the oxidative stress conditions that these infants experience [14]. Preterm infants exposed to elevated oxygen levels early in life generate excessive reactive oxygen species, which can overwhelm their limited antioxidant defenses and contribute to a range of severe neonatal conditions [7, 15, 16]. Hemoglobin F (HbF), which is the primary oxygen-carrying hemoglobin in VLBW infants at birth and in the early postnatal period, decreases rapidly due to iatrogenic blood loss and transfusions, while the relative proportion of HbA increases [8, 17]. Unlike HbA, HbF demonstrates enhanced pseudo-peroxidase activity, which facilitates the neutralization of peroxides and the scavenging of free radicals in other molecules [5–7]. Torrejón-Rodríguez et al. observed that preterm infants with lower HbF levels early in life exhibit elevated oxidative stress biomarkers(14). Recent studies have further established that persistently low average HbF levels during hospitalization correlate with the development and severity of oxidative stress-related conditions(17). Beyond oxidative stress, the rapid decline in HbF levels may also be linked to the loss of endogenous blood components. The decline in circulating insulin-like growth factor 1 (IGF-1) levels parallels the swift decrease in HbF early in life, and persistently low IGF-1 levels have been associated with the development of BPD, retinopathy of prematurity, and postnatal growth restriction [18–20]. Ley et al. demonstrated that early supplementation with endogenous blood components, such as recombinant IGF-1 (rhIGF1) and recombinant IGF-binding protein 3 (rhIGFBP-3), can mitigate the severity of BPD[21, 22].
This study utilized threshold analysis [13, 23] to advance our understanding of the relationship between HbF levels and the incidence of BPD, extending previous qualitative research that established an association between these factors. By precisely defining risk thresholds, this analysis offers a quantitative framework for predicting BPD with greater accuracy. This advancement provides clinicians with a robust tool for risk assessment, thereby enhancing the precision of BPD predictions and supporting more informed decision-making in clinical practice.
Based on the theories outlined above, moderating the rate of HbF decline in the early postnatal period may provide a protective effect in preterm infants with underdeveloped antioxidant systems, potentially mitigating the onset of free radical-related pathologies. Elevated HbF levels in premature infants may be related to maternal hypertension [24]. A significant reduction in iatrogenic blood loss could have a substantial impact on the levels of circulating growth factors. Additionally, interventions such as delayed cord clamping and umbilical cord milking, which can elevate HbF levels, may also aid in preserving endogenous blood components. Future research is needed to explore whether early administration of antioxidant agents, including vitamin A and E, zinc, or Melatonin, could effectively reduce oxidative stress and prevent associated damage during this critical period of rapid HbF decline [25].
This study identified a trend of increased HbF levels early in life among some very low birth weight infants, particularly those who did not receive a blood transfusion within the first 7 days. M. S. Brown et al. observed that HbF levels in very low birth weight infants temporarily rise following the cessation of frequent transfusions [18]. Wilson K et al. found that the HbF ratio is 0.82 in full-term infants, 0.89 in preterm infants born at 34–37 weeks, and 0.92 in preterm infants born at ≤ 32 weeks, with extremely preterm infants maintaining elevated HbF ratios throughout [26]. This suggests that the increase in HbF levels postnatally may be related to ongoing HbF production, as well as improvements in blood sampling techniques, reduced iatrogenic blood loss, and decreased transfusion requirements [27].
In this study, fetal hemoglobin (HbF) levels were quantified using a blood gas analyzer integrated with a co-oximetry module, which operates on the principle of multi-wavelength spectrophotometric analysis. This approach offers several advantages for routine clinical application, including high efficiency, full automation, and minimal sample volume requirements, thereby enabling reliable and practical monitoring of HbF levels in neonatal populations.
This study has several limitations. Firstly, the sample was obtained from a single center, which limits the generalizability of the findings. To strengthen the evidence, there is a need for multicenter studies with larger sample sizes to further validate the relationship between early postnatal HbF decline and the risk of BPD in very low birth weight infants. Additionally, this study only assesses the relationship between HbF decline within the first 7 days of life and the development of BPD. Future research should address these extended time points and explore how HbF decline over a longer period correlates with BPD risk. Such studies would provide a more comprehensive understanding and support the development of targeted interventions in diverse clinical settings. Finally, although both HbF decline value and decline rate were used as parallel indicators in the analysis, only the decline rate yielded extreme odds ratios. This may be due to its small unit magnitude combined with considerable variability in the data, which could have inflated the estimated effect sizes and potentially led to instability in the results.
Conclusions
The incidence of mortality and/or BPD in very low birth weight infants is non-linearly associated with HbF decline values or rates during the first 7 days of life. Specifically, when the HbF decline value surpasses 3 g/L or the decline rate exceeds 0.05, there is an increased risk of both mortality and/or BPD. In clinical practice, HbF levels can serve as a valuable predictive marker, which, when combined with other relevant factors, can facilitate timely and well-informed decision-making.
Acknowledgements
Not applicable.
Authors’ contributions
CY.N. performed study concept and design, performed development of methodology, analysis and interpretation of data, writing– original draft, read and approved the final paper.H.W. performed study concept and design, provided technical and material support, read and approved the final paper.L.Z. analysis and interpretation of data, statistical analysis, read and approved the final paper.D. D. provided technical support, read and approved the final paper. XP. L. analysis and interpretation of data, statistical analysis, read and approved the final paper.X. M. performed study concept and design, project administration, writing– review & editing, read and approved the final paper.
Funding
Jilin Provincial Department of Science and Technology (YDZJ202301ZYTS070).
Data availability
The authors declare that all data supporting the findings of this study are available within the manuscript and its supplementary information files.
Declarations
Ethics approval and consent to participate
Ethics review board of the First Hospital of Jilin University (23K005-001).
Consent for publication
Written informed consent was not required for retrospective observational study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ. 2021;375: n1974. [DOI] [PubMed] [Google Scholar]
- 2.Hwang JS, Rehan VK. Recent advances in bronchopulmonary dysplasia: pathophysiology, prevention, and treatment. Lung. 2018;196:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorente-Pozo S, Parra-Llorca A, Lara-Cantón I, Solaz A, García-Jiménez JL, Pallardó FV, Vento M. Oxygen in the neonatal period: oxidative stress, oxygen load and epigenetic changes. Semin Fetal Neonatal Med. 2020;25: 101090. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Dong W. Oxidative stress and bronchopulmonary dysplasia. Gene. 2018;678:177–83. [DOI] [PubMed] [Google Scholar]
- 5.Bard H. Postnatal fetal and adult hemoglobin synthesis in early preterm newborn infants. J Clin Invest. 1973;52:1789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felicetti L, Novelletto A, Benincasa A, Terrenato L, Colombo B. The HbA/HbA2 ratio in newborns and its correlation with fetal maturity. Br J Haematol. 1984;56:465–71. [DOI] [PubMed] [Google Scholar]
- 7.Petschow R, Petschow D, Bartels R, Baumann R, Bartels H. Regulation of oxygen affinity in blood of fetal, newborn and adult mouse. Respir Physiol. 1978;35:271–82. [DOI] [PubMed] [Google Scholar]
- 8.De Halleux V, Truttmann A, Gagnon C, Bard H. The effect of blood transfusion on the hemoglobin oxygen dissociation curve of very early preterm infants during the first week of life. Semin Perinatol. 2002;26:411–5. [DOI] [PubMed] [Google Scholar]
- 9.Hellström W, Martinsson T, Hellstrom A, Morsing E, Ley D. Fetal haemoglobin and bronchopulmonary dysplasia in neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2021;106:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merz U, Peschgens T, Hörnchen H. [Fiberoptic measurement of arterial oxygen saturation in premature and term neonates]. Z Geburtshilfe Neonatol. 1999;203:77–80. [PubMed] [Google Scholar]
- 11.Shepherd Ali G, Ashoka Ali A, Steinke JM, Shepherd AP. A simple method for measuring fetal hemoglobin by co-oximetry. Clin Chim Acta. 2001;307:249–52. [DOI] [PubMed] [Google Scholar]
- 12.Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, Kirpalani H, Laughon MM, Poindexter BB, Duncan AF, Yoder BA, Eichenwald EC, DeMauro SB. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu X, Cao L, Yu X. Elevated cord serum manganese level is associated with a neonatal high ponderal index. Environ Res. 2013;121:79–83. [DOI] [PubMed] [Google Scholar]
- 14.Torrejón-Rodríguez L, Parra-Llorca A, Pinilla-González A, Lara-Cantón I, Albiach-Delgado A, Cernada M, Escrig R, Kuligowski J, Aguar Carrascosa M, Torres V, M. Do lower levels of fetal hemoglobin in preterm infants relate to oxidative stress?? Antioxid Redox Signal. 2024;40:453–9. [DOI] [PubMed] [Google Scholar]
- 15.Jiramongkolchai K, Repka MX, Tian J, Aucott SW, Shepard J, Collins M, Clemens J, Feller M, Burd I, Roizenblatt M, Smith K, Arevalo JF, Gehlbach PL, Handa JT. Effects of fetal haemoglobin on systemic oxygenation in preterm infants and the development of retinopathy of prematurity PacIFiHER report 2. Br J Ophthalmol. 2023;107:380–3. [DOI] [PubMed] [Google Scholar]
- 16.Prasad N, Dubey A, Kumar K, Shrivastava J. Role of fetal hemoglobin in the development and progression of retinopathy of prematurity in preterm infants. Indian J Ophthalmol. 2023;71:3478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stutchfield CJ, Jain A, Odd D, Williams C, Markham R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: a pilot prospective cohort study. Eye (Lond). 2017;31:1451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown MS, Phipps RH, Dallman PR. Postnatal changes in fetal hemoglobin, oxygen affinity and 2,3-diphosphoglycerate in previously transfused preterm infants. Biol Neonate. 1985;48:70–6. [DOI] [PubMed] [Google Scholar]
- 19.Hellström A, Ley D, Hansen-Pupp I, Hallberg B, Löfqvist C, van Marter L, van Weissenbruch M, Ramenghi LA, Beardsall K, Dunger D, Hård AL, Smith LE. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 2016;105:576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellström A, Ley D, Hansen-Pupp I, Hallberg B, Ramenghi LA, Löfqvist C, Smith LE, Hård AL. Role of insulinlike growth factor 1 in fetal development and in the early postnatal life of premature infants. Am J Perinatol. 2016;33:1067–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung JK, Hallberg B, Hansen-Pupp I, Graham MA, Fetterly G, Sharma J, Tocoian A, Kreher NC, Barton N, Hellström A, Ley D. Development and verification of a pharmacokinetic model to optimize physiologic replacement of rhIGF-1/rhIGFBP-3 in preterm infants. Pediatr Res. 2017;81:504–10. [DOI] [PubMed] [Google Scholar]
- 22.Ley D, Hallberg B, Hansen-Pupp I, Dani C, Ramenghi LA, Marlow N, Beardsall K, Bhatti F, Dunger D, Higginson JD, Mahaveer A, Mezu-Ndubuisi OJ, Reynolds P, Giannantonio C, van Weissenbruch M, Barton N, Tocoian A, Hamdani M, Jochim E, Mangili A, Chung JK, Turner MA, Smith LEH, Hellström A. rhIGF-1/rhIGFBP-3 in preterm infants: A phase 2 randomized controlled trial. J Pediatr. 2019;206:56–e6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Jia F, Li C, Yuan H, Yang H, Yang R, Yue Y, Zhang G, Zhang X, Ye G, Li Z, Du X, Zhang X. Association between body mass index and suicide attempts in Chinese patients of a hospital in Shanxi district with first-episode drug-naïve major depressive disorder. J Affect Disord. 2023;339:377–83. [DOI] [PubMed] [Google Scholar]
- 24.Masoumi Z, Familari M, Källén K, Ranstam J, Olofsson P, Hansson SR. Fetal hemoglobin in umbilical cord blood in preeclamptic and normotensive pregnancies: a cross-sectional comparative study. PLoS One. 2017;12:e0176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrante G, Montante C, Notarbartolo V, Giuffrè M. Antioxidants: role the in prevention and treatment of bronchopulmonary dysplasia. Paediatr Respir Rev. 2022;42:53–8. [DOI] [PubMed] [Google Scholar]
- 26.Wilson K, Hawken S, Murphy MSQ, Atkinson KM, Potter BK, Sprague A, Walker M, Chakraborty P, Little J. Postnatal prediction of gestational age using newborn fetal hemoglobin levels. EBioMedicine. 2017;15:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bednarczuk N, Williams EE, Kaltsogianni O, Greenough A, Dassios T. Postnatal temporal changes of foetal haemoglobin in prematurely born infants. Acta Paediatr. 2022;111:1338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the manuscript and its supplementary information files.