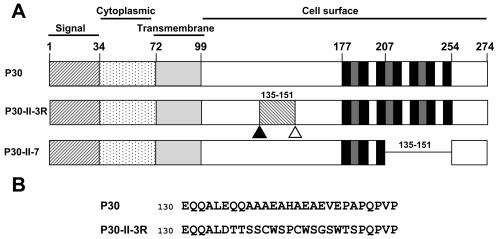
FIG. 1.
Cytadherence-associated protein P30 in wild-type M. pneumoniae, mutants II-3 and II-7, and revertant II-3R. (A) Wild-type P30 is predicted to have a single transmembrane domain. Experimental data (10, 32) suggest an orientation with the C terminus on the cell surface. For P30-II-3R, a frameshift (open triangle) resulted in mutant II-3 with no detectable P30. A second site mutation (solid triangle) in revertant II-3R restored P30 except for residues 135 to 151 (see panel B below). P30-II-7 is a P30 derivative resulting from in-frame deletion of residues 207 to 254 in mutant II-7. (B) Amino acid sequence for P30 from wild-type and II-3R M. pneumoniae for the indicated residues.