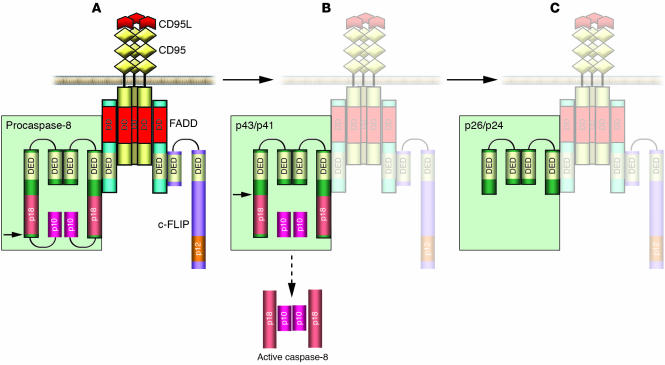
Figure 2.
Scheme of procaspase-8 processing at the CD95 DISC. CD95 DISC formation is triggered by extracellular cross-linking with CD95L (depicted in red), which is followed by oligomerization of the receptor. FADD/MORT1 is recruited to the DISC by DD interactions (shown in red); procaspase-8 and -10 as well as c-FLIP proteins are recruited to the DISC by homophilic DED interactions (yellow). Upon recruitment to the DISC, procaspase-8 undergoes processing by forming dimers (depicted in green). (A) The first step of procaspase-8 cleavage occurs between 2 protease subunits. The site of cleavage is shown by a black arrow. As a result of the first cleavage step the p10 subunit is formed, which is not released into the cytosol but remains bound to the DISC as it is involved in the interactions with the large protease subunits. (B) The second cleavage step takes place between the prodomain and the large protease subunit at Asp216. As a result of this cleavage step the active caspase-8 heterotetramer is formed, which is then released into the cytosol. (C) Prodomain p26/p24 remains bound to the DISC.