Fig. 1. Initiation of Na+-glucose cotransport results in increased ezrin phosphorylation at threonine 567 and a corresponding rise in Akt phosphorylation at both threonine 308 and serine 473.
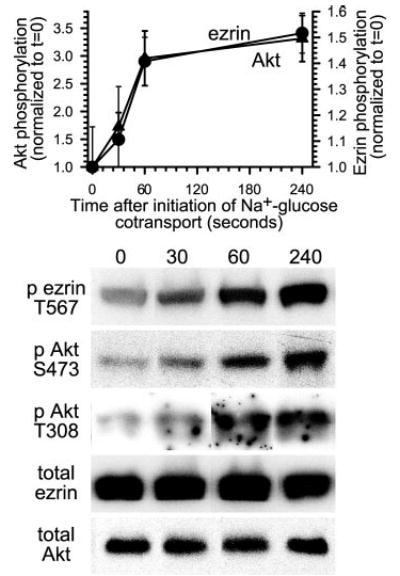
Monolayers were lysed at indicated times after initiation of Na+-glucose cotransport, and lysates were immunoblotted for threonine 567 phosphorylated ezrin, total ezrin, threonine 308 phosphorylated Akt, serine 473 phosphorylated Akt, and total Akt. Densitometric analysis of triplicate samples from this experiment are shown and represent the mean and S.D. of the ratios of threonine 567 phosphorylated ezrin to total ezrin (circles) and serine 473 phosphorylated Akt to total Akt (triangles). These ratios increased by 52 ± 3% and 231 ± 29%, respectively, at 240 s after initiation of Na+-glucose cotransport (p < 0.01). Increases in threonine 308 Akt phosphorylation were similar to serine 473 phosphorylation. Representative immunoblots are shown. Results are typical of at least three independent experiments, each with triplicate samples.
