Fig. 2. Inhibition of p38 MAP kinase prevents both ezrin and Akt activation.
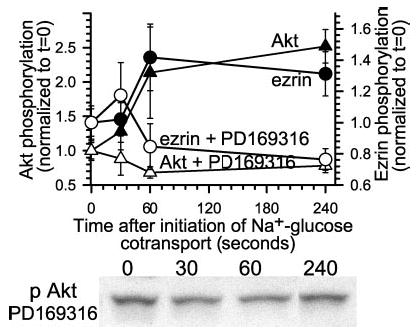
Monolayers were pretreated with the p38 MAP kinase inhibitor PD169316 (10 μm) and lysed at indicated times after initiation of Na+-glucose cotransport. Lysates were immunoblotted for threonine 567 phosphorylated ezrin, total ezrin, serine 473 phosphorylated Akt, and total Akt. Rather than a 152 ± 15% increase in serine 473 Akt phosphorylation 240 s after initiation of Na+-glucose cotransport (black triangles), serine 473 Akt phosphorylation decreased 22 ± 3% in PD169316-treated monolayers (white triangles). Likewise, as we have reported previously (25), p38 MAP kinase inhibition blocked increases in ezrin phosphorylation after initiation of Na+-glucose cotransport (black and white circles). Representative immunoblots of Akt phosphorylated at serine 473 are shown. Results are typical of more than three independent experiments, each with triplicate samples.
