Fig. 7. Akt activity is required for NHE3 translocation and cytoplasmic alkalinization after Na+-glucose cotransport.
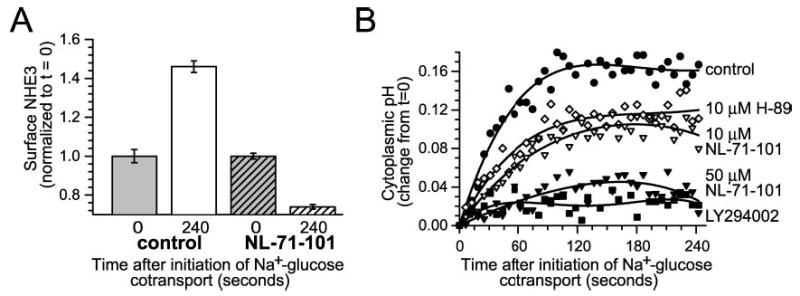
A, Akt activity is necessary for ezrin translocation after initiation of Na+-glucose cotransport. Monolayers were fixed 240 s after initiation of Na+-glucose cotransport in the absence or presence of 50 μm NL-71-101. Surface NHE3 was assessed by surface immunofluorescence of nonpermeabilized cells, as described previously (25). 50 μm NL-71-101 completely prevented increases in surface NHE3 expression after initiation of Na+-glucose cotransport (p < 0.01). Results are typical of three independent experiments, each with triplicate samples in which each sample was evaluated over at least 10 separate fields. B, Akt activity is required for NHE3-dependent cytoplasmic alkalinization. Pharmacological inhibition of Akt with 50 μm NL-71-101 (black inverted triangles) or of PI3 kinase with LY294002 (black squares) almost completely prevented NHE3-dependent cytoplasmic alkalinization after initiation of Na+-glucose cotransport (p < 0.01). In contrast, 10 μm H-89 (white diamonds) or 10 μm NL-71-101 (white inverted triangles) only partially inhibited cytoplasmic alkalinization, paralleling their incomplete inhibition of ezrin phosphorylation in vitro (see Fig. 4). Results are typical of five independent experiments.
