Figure 5.
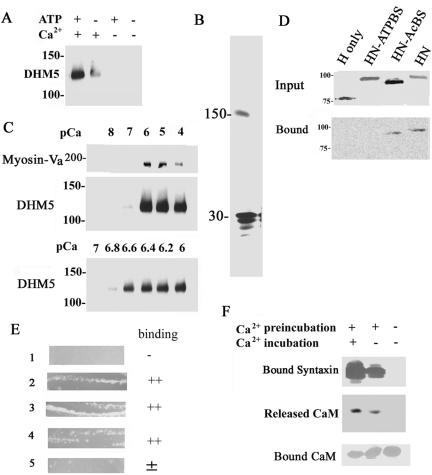
The syntaxin-1A-binding site is located in the neck domain of myosin-Va. (A) Recombinant myosin-Va without the globular tail (DHM5) can bind syntaxin-1A in a Ca2+-dependent manner in the presence of and MgATP. DHM5 was incubated with GST-syntaxin-1A in the presence and absence of 10–6 M Ca2+ and with or without MgATP. DHM5 binding was detected by immunoblotting with an antibody against anti-myosin-Va head. The binding specificity of the recombinant tailless myosin-Va (DHM5) is similar to the full-length brain myosin-Va (see Figure 1D). (B) Syntaxin-1A binds to immobilized tailless myosin-Va (DHM5). Syntaxin-1A [1-262] (30-kDa) was incubated with Ni2+-NTA-immobilized His6-DHM5 in the presence of 10–6 M Ca2+ (see Figure 1C) and eluted with SDS-sample buffer. The eluted sample was separated by SDS-PAGE and silver stained. The 150-kDa band is DHM5. (C) The binding of truncated myosin-Va (DHM5) by syntaxin-1A requires a pCa of 6.6. Syntaxin-1A [1-262] was incubated with DHM5 and the purified myosin-Va from brain in the presence of various concentrations of Ca2+. Binding of myosin-Va and DHM5 was detected by immunoblotting with an antibody against myosin-Va. Top and middle, dose response of Ca2+ for binding of syntaxin-1A by DHM5 between pCa 4 and 8. Brain myosin-Va (Myosin-V) and DHM5 show a similar dependence on Ca2+ for the binding of syntaxin-1A. Bottom, dose response of Ca2+ for binding of syntaxin-1A by DHM5 between pCa 6 and 7. (D) The neck domain of myosin-Va is necessary for syntaxin-1A binding. Truncated forms of myosin-Va were produced by biotin-labeled in vitro translation of mouse myosin-Va cDNA (dilute): H, head domain only (amino acids 1–755); HN-ATPBS, head and neck domains (amino acids 1–911) lacking the ATP-binding site (amino acids 164–171); HN-AcBS, head and neck domain lacking the actin-binding site (amino acids 643–666); and HN, head and neck domains (Espreafico et al., 1992). Top, in vitro-translated proteins. Bottom, binding of these constructs to syntaxin-1A in the presence of 10–6 M Ca2+ and 0.5 mM ATP. Unlike the head domain alone, the head-and-neck portion can bind to syntaxin-1A. Note that the ATP-binding site but not the actin-binding site is also necessary for the binding. The biotin-labeled in vitro-translated proteins were visualized by the streptavidin-conjugated alkaline phosphatase. (E) A bacterial two-hybrid assay (BacterioMatch) reveals that the neck domain of myosin-Va contains the syntaxin-1A-binding site. Reporter strains of Escherichia coli (Stratagene) were cotransfected with myosin-Va and syntaxin-1A constructs and incubated at 30°C for 24 h. Lane 1, pBT+pTRG (manufacturer's negative control); lane 2, pBT-LGF2 + pTRG-GAL11p (manufacturer's positive control); lane 3, pBT-6IQ (amino acids 764–908 of mouse myosin-Va) + pTRG-CaM; lane 4, pBT-6IQ + pTRG-syntaxin-1A [1-262]; lane 5, pBT-1IQ (amino acids 764–787 of mouse myosin Va) + pTRG-syntaxin-1A [1-262]. Lanes 2–4 were judged to be the binding-positive plates. (F) Ca2+ requirement for binding of syntaxin-1A to myosin-V is due to Ca2+-dependent release of CaM from the neck domain of myosin-Va. Immobilized His6-DHM5, which copurified with CaM from Sf9 cells (Homma et al., 2000), was first preincubated for 1 h with phosphate-buffered saline (PBS) containing 10–6 M Ca2+ (Ca2+-phosphate-buffered saline; +) or with Ca2+-free PBS (–). The His6-DHM5-CaM complex was then incubated for another 1 h with syntaxin-1A in Ca2+-phosphate-buffered saline (+) or Ca2+-free PBS (–). Top, detection of syntaxin-1A binding by immunoblotting. After the preincubation with Ca2+, which released CaM from DHM5, Ca2+ was no longer necessary for the binding of syntaxin-1A by DHM5. Immunoblotting for CaM confirmed that, after the preincubation with Ca2+, CaM was released into the supernatant (middle) and no longer bound to DHM5 (bottom).