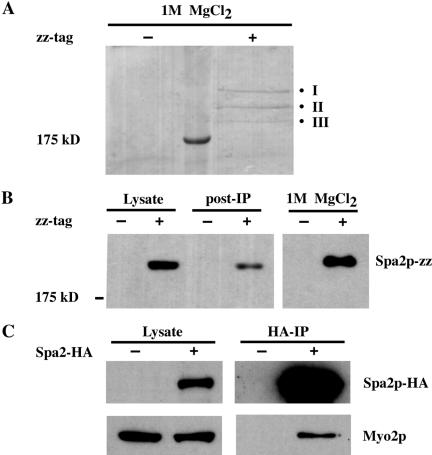
Figure 1.
Immunoprecipitation of proteins that interact with Spa2-zz. (A) Lysates derived from untagged (JSY162) or Spa2-zz (JSY163) strains were incubated with IgG-Sepharose beads for 90 min at 4°C. The resin was washed, and bound proteins were eluted with 1 M MgCl2. Eluted proteins were concentrated, separated on 6.5% SDS-PAGE, and visualized by Coomassie Blue staining. Three bands (I, II, and III) were present specifically in the Spa2-zz immunoprecipitate and individually excised for tandem mass spectrometry analysis. (B) Samples of the lysates before and after (post-IP) incubation with IgG-Sepharose beads and the 1 M MgCl2 eluant were separated by SDS-PAGE, transferred to nitrocellose, and visualized by immunoblotting with preimmune serum. (C) Myo2p coimmunoprecipitates with Spa2p-HA. Proteins prepared from untagged (JSY162) and Spa2p-HA (JSY214) strains were immunoprecipitated with HA-Sepharose beads for 2 h at 4°C. Total lysates and immunoprecipitates were analyzed on immunoblots and probed with anti-HA or anti-Myo2p antibodies.