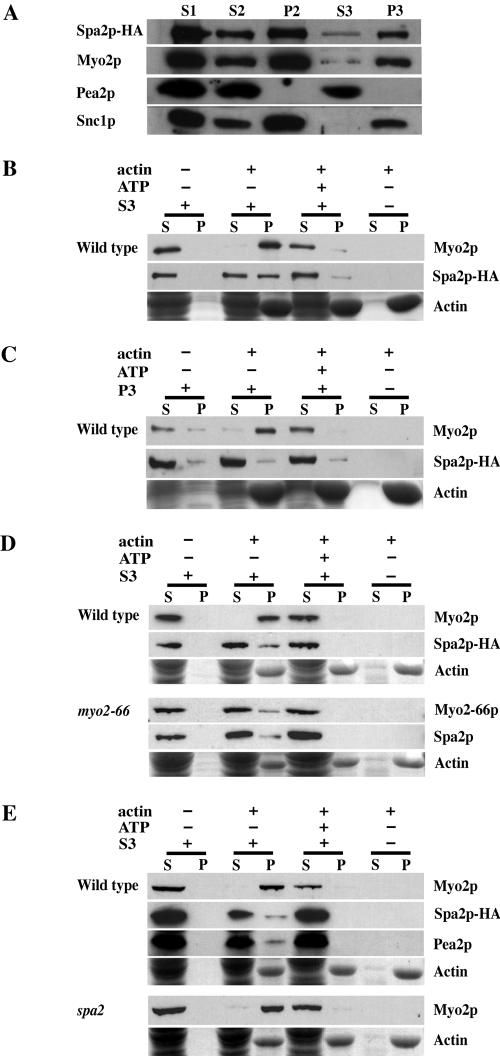
Figure 2.
(A) Spa2p cofractionates with Myo2p. Lysate from logphase cells of the Spa2-HA strain (JSY214) was spun at 2000 × g to generate supernatant 1 (S1). S1 was spun at 30,000 × g, resulting in S2 and pellet 2 (P2). S2 was spun at 100,000 × g to generate S3 and P3. Gel samples were prepared using volumetric stoichiometry and equal volumes of each sample were loaded per lane, separated by SDS-PAGE, and transferred to nitrocellulose. Blots were then probed with anti-HA, anti-Myo2p, anti-Pea2p, or anti-Snc1p antibodies. P2 is the plasma membrane fraction, as Pma1p, a marker of the plasma membrane in yeast, is found primarily in P2 (Reck-Peterson et al., 1999). P3 is the microsomal fraction and is enriched in late secretory vesicles (Goud et al., 1988). Snc1p, a late vesicleborne, integral membrane protein (Protopopov et al., 1993), is present primarily in the P3 fraction. (B–E) Actin cosedimentation assays. S3 supernatant or P3 pellet fractions derived from lysates of wild-type strains, NY580 (B and C), JSY278 (D) or JSY261 (E); myo2-66 strain, JSY279, (D); or spa2Δ strain, JSY262 (E) were used for actin cosedimentation assays. Wild-type and myo2-66 strains in D were grown at 25°C and shifted to 37°C for 2.5 h before harvesting and cell lysis. Myo2p, Myo2-66p, Spa2p-HA, and Pea2p present in the S3 (B, D, and E) or Myo2p and Spa2p-HA present in the P3 (C) were incubated with F-actin in the presence or absence of 4 mM ATP. After centrifugation to pellet the F-actin, the supernatant and pellet fractions were immunoblotted with antibodies to Myo2p, HA or Pea2p. Actin was visualized by Coomassie Blue staining. A fraction of Spa2p and Pea2p (present in S3) cosediment with actin in an ATP-sensitive manner similar to that of Myo2p.