Abstract
The mechanism of AP-1/clathrin coat formation was analyzed using purified adaptor proteins and synthetic liposomes presenting tyrosine sorting signals. AP-1 adaptors recruited in the presence of Arf1·GTP and sorting signals were found to oligomerize to high-molecular-weight complexes even in the absence of clathrin. The appendage domains of the AP-1 adaptins were not required for oligomerization. On GTP hydrolysis induced by the GTPase-activating protein ArfGAP1, the complexes were disassembled and AP-1 dissociated from the membrane. AP-1 stimulated ArfGAP1 activity, suggesting a role of AP-1 in the regulation of the Arf1 “GTPase timer.” In the presence of cytosol, AP-1 could be recruited to liposomes without sorting signals, consistent with the existence of docking factors in the cytosol. Under these conditions, however, AP-1 remained monomeric, and recruitment in the presence of GTP was short-lived. Sorting signals allowed stable recruitment and oligomerization also in the presence of cytosol. These results suggest a mechanism whereby initial assembly of AP-1 with Arf1·GTP and ArfGAP1 on the membrane stimulates Arf1 GTPase activity, whereas interaction with cargo induces oligomerization and reduces the rate of GTP hydrolysis, thus contributing to efficient cargo sorting.
INTRODUCTION
Intracellular transport between membrane compartments is initiated by the recruitment of cytosolic coat proteins, which perform several functions (Kirchhausen, 2000; Aridor and Traub, 2002). They select and concentrate cargo proteins, polymerize to form a lattice structure on the membrane surface, and deform the lipid bilayer to bud toward the cytosol. On completion of a coated vesicle, the coat components disassemble, allowing fusion with the target compartment. The three best characterized coats are coat protein (COP) I mediating intra-Golgi and Golgi-to-endoplasmic reticulum transport, COPII for vesicles derived from the endoplasmic reticulum, and clathrin with various associated adaptor proteins for pathways between the plasma membrane, endosomes, and the trans-Golgi network (Kirchhausen, 2000).
In all systems (apparently even for clathrin-dependent endocytosis; Paleotti et al., 2005), coat recruitment is initiated by a small GTPase that is activated at the membrane by a guanine nucleotide exchange factor (GEF). The minimal requirements to form coats have been defined in vitro using chemically defined liposomes and purified coat components. The generation of COPI vesicles required the heteroheptameric coatomer complex and ADP-ribosylation factor 1 (Arf1) and was enhanced by acidic phospholipids or by lipid-anchored sorting signals (Spang et al., 1998; Bremser et al., 1999). COPII consists of two dimers that can be sequentially assembled on liposomes containing phosphoinositides. Sec23/24 is first targeted by Sar1·GTP to the membrane as a primer to recruit the second layer of Sec13/31 (Matsuoka et al., 1998a, 1998b). Clathrin coats are similarly composed of two layers (Robinson and Bonifacino, 2001). Typically, heterotetrameric adaptor proteins (APs) connect cargo molecules in the membrane with the outer layer of clathrin triskelia. AP-3, Arf1·GTP, and clathrin were sufficient to produce coats and clathrin-coated vesicles (CCVs; Drake et al., 2000). In contrast, AP-1 recruitment to liposomes and CCV formation required cytosolic factor(s) in addition to Arf1·GTP (Zhu et al., 1999). Alternatively, AP-1 recruitment could be reconstituted in the absence of cytosol on liposomes presenting covalently linked sorting signals (Crottet et al., 2002).
GTP hydrolysis causes uncoating of COPI and COPII coats (Tanigawa et al., 1993; Antonny et al., 2001). Sar1 and Arf1 have low intrinsic GTPase activity, and specific GTPase-activating proteins (GAPs) act in a regulated manner to obtain an appropriately timed deactivation of these G proteins (Randazzo and Hirsch, 2004). The GAP for Sar1 is Sec23, i.e., a subunit of the first COPII layer. Recruitment of the second subcomplex, Sec13/31, further stimulates GAP activity, thus accelerating disassembly of the completed coat (Antonny et al., 2001).
Arf GAPs are a family of proteins containing a conserved catalytic domain, whereas other parts of the proteins are highly variable (Randazzo and Hirsch, 2004). Two types of Arf1 GAPs, ArfGAP1, and ArfGAP2/3 (Gcs1 and Glo3 in Saccharomyces cerevisiae) were implicated in Golgi trafficking (Poon et al., 1999; Yang et al., 2002; Lewis et al., 2004; Watson et al., 2004). GTP hydrolysis in COPI coats is activated by ArfGAP1 (Cukierman et al., 1995) and was shown to contribute to cargo sorting, in addition to uncoating (Nickel et al., 1998; Lanoix et al., 1999; Malsam et al., 1999; Pepperkok et al., 2000). Coatomer was found to stimulate ArfGAP1-mediated GTP hydrolysis on Arf1 (Goldberg, 1999). This stimulation was inhibited by peptides derived from specific COPI cargo (hp24a/p24β1), suggesting a mechanism for increasing the probability for cargo to be incorporated into the growing coat polymer (Goldberg, 2000; Lanoix et al., 2001; Weiss and Nilsson, 2003).
Little is known about the role of GTP hydrolysis in clathrin coats with AP-1 or AP-3. In purified CCVs, almost no Arf1 could be detected, suggesting that GTP hydrolysis is not per se sufficient to induce disassembly of the complete coat (Zhu et al., 1998). Here we have studied the role of sorting signals and GTP hydrolysis in the recruitment of AP-1 adaptors to liposomes. Our findings show that cargo signals cause AP-1 to form high-molecular-weight complexes even in the absence of clathrin. These complexes are susceptible to GTP hydrolysis induced by ArfGAP1. AP-1 regulates the activity of ArfGAP1, indicating that controlled GTP hydrolysis may play a role in cargo selection and productive coat formation.
MATERIALS AND METHODS
Protein Purification
CCVs were isolated from calf brains, the coats were released, and mixed APs were purified as described (Crottet et al., 2002). To isolate AP-1 adaptors, mixed APs were dialyzed into 20 mM ethanolamine, pH 8.9, 2 mM EDTA, 1 mM dithiothreitol (DTT; MonoQ buffer), loaded on a MonoQ HR 5/5 (Amersham Biosciences, Piscataway, NJ) and eluted by a 5-ml linear gradient of 0–150 mM NaCl followed by a 50-ml linear gradient of 150–450 mM NaCl in starting buffer (adapted from Ahle et al., 1988). AP-1 containing fractions free of ArfGAP1 (as judged by immunoblotting) were pooled and subjected to hydroxyapatite chromatography as described (Ahle and Ungewickell, 1986; Crottet et al., 2002). Myristoylated Arf1 was purified as described by Liang and Kornfeld (1997) and for GAP assays as described by Franco et al. (1995). His6-tagged full-length ArfGAP1 and the catalytic domain (residues 1–136) were expressed in baculovirus-infected Sf9 cells and Escherichia coli BL21 (DE3) cells, respectively (Huber et al., 2001). Coatomer was purified from rabbit liver as described by Pavel et al. (1998).
To produce AP-1 complexes lacking the appendage domains of γ- and β1-adaptins, AP-1 was purified by separating coat proteins released from CCVs by hydroxyapatite chromatography (Ahle and Ungewickell, 1986) followed by dialysis of AP-1-containing fractions into MonoQ buffer, and ion exchange chromatography on a MonoQ HR10/10 column eluted by a 10-ml gradient of 0–150 mM NaCl and a 100-ml gradient of 150–450 mM NaCl in MonoQ buffer. Purified AP-1 at 10 μg/ml in MonoQ buffer was digested with TPCK-treated trypsin (Sigma, Buchs, Switzerland) at an enzyme/substrate weight ratio of 1:1 for 30 min at room temperature to remove the β1 and most γ appendages, but leaving μ1 intact (Schröder and Ungewickell, 1991). Stronger trypsin treatment led to partial digestion of μ1, resulting in reduced liposome recruitment (unpublished data). Reactions were stopped on ice with a fivefold excess of ovomucoid (trypsin inhibitor; Sigma). The extent of digestion was monitored by immunoblot analysis using mouse anti-γ-adaptin (100/3 from E. Ungewickell, Hannover, Germany) directed against the hinge sequence to detect the undigested γ subunit, mouse anti-β1/2-adaptin (100/1; Sigma) recognizing an epitope in the β core, and a rabbit anti-μ1A antiserum raised against the synthetic peptide EAEDKEGKPPISV.
Liposome Recruitment Assay
Peptidoliposomes made of 97.5% soybean phospholipids (a mixture of phospholipids also containing phosphoinositides, sold as azolectin by Sigma; Zhu et al., 1999) and 2.5% N-((4-maleimidylmethyl)cyclohexane-1-carbonyl)-1,2-dipalmitoyl- or -dioleoyl-sn-glycero-3-phosphoethanolamine (MMCC-DPPE [Molecular Probes, Eugene, OR] and MMCC-DOPE [Avanti Polar Lipids, Alabaster, AL]) were obtained after extrusion through 400-nm pore size polycarbonate filters and incubation with synthetic peptides CRKRSHAGYQTI (LY) or CRKRSHAGAQTI (LA; Crottet et al., 2002). Recruitment assays were performed essentially as described (Crottet et al., 2002). In brief, 100 μl of peptidoliposomes (0.5 μmol lipid) were incubated for 30 min at 37°C with 5 μg of Arf1, 0.2 mM GMP-PNP or 2 mM GTP, and 10 μg of mixed adaptors or 0.5 μg of pure AP-1. Samples were loaded at the bottom of a sucrose step gradient and centrifuged at 300,000 × gav for 1 h at 4°C to float the liposomes. To test the effect of GTP hydrolysis, half the top fraction (500 μl) was incubated with 10 μg ArfGAP1 at 37°C for 30 min before a second floatation. Fractions were analyzed by trichloroacetic acid precipitation, SDS-PAGE, and immunoblotting using antibodies against γ-adaptin (100/3) or Arf1 (1D9 from Alexis, Lausen, Switzerland), a peroxidase-coupled secondary antibody (Sigma), and enhanced chemiluminescence.
Calf brain cytosol was prepared as before (Crottet et al., 2002) and centrifuged for 30 min at 170,000 × g immediately before use. Liposomes (0.5 μmol lipid) with or without coupled peptides were incubated for 30 min at 37°C with 1 mg of cytosol, 5 μg of Arf1 (or Arf1Q71L, a gift by K. Fiedler), and 0.2 mM GMP-PNP or 2 mM GTP in a total volume of 175 μl and analyzed as above.
Velocity Sedimentation
Floated liposomes (340 μl) were mixed with 340 μl assay buffer, supplemented with Triton X-100 or octylglucoside (Sigma) to 0.5%, loaded onto 4.3 ml of a 10–25% sucrose gradient in assay buffer with 0.2% Triton or octylglucoside, and centrifuged at 100,000 × gav for 5 h at 4°C. Where indicated, solubilization was performed at 37°C and centrifugation at room temperature. Ten 0.5-ml fractions were collected from the top and analyzed by immunoblotting. Rat IgM (a gift by A. Rolink, University of Basel) and ribosomes prepared from bovine adrenals (Brown et al., 1974) were used as standards. In a control experiment, 2.5% N-((6-(biotinoyl)amino)hexanoyl)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (biotin-DPPE; Molecular Probes) was in addition incorporated into the liposomes, and an AP-1 recruitment experiment was performed in the presence of 20 μg FITC-streptavidin (from Serotech, Basel, Switzerland) followed by floatation, solubilization, and velocity sedimentation as above. FITC-streptavidin was quantified by fluorimetry.
GTPase Assay
Assays were performed essentially as described (Huber et al., 2001). Arf1 (4 μM) was loaded with [γ-32P]GTP (2.5 μM at ∼100 Ci/mmol; from NEN, Geneva, Switzerland) by incubation with peptidoliposomes (1 mg soybean phospholipids/ml) in 25 mM MOPS, pH 7.5, 100 mM KCl, 1 mM MgCl2, 2 mM EDTA for 15 min at 30°C, and loading was terminated by addition of 2 mM MgCl2. Loading efficiency was typically 40–50% of initial [γ-32P]GTP as determined by filtration through a 0.45-μm nitrocellulose filter. To diminish inorganic [32P]phosphate background, the sample was centrifuged at 20,000 × g for 20 min at 4°C, and the liposome pellet was resuspended in 25 mM MOPS, pH 7.5, 5 mM MgCl2, 40 mM KCl, 1 mM DTT. [γ-32P]GTP-loaded Arf1, 40 nM, was preincubated for 5 min at 30°C in 25 μl of the same buffer with or without 0.25 μM coatomer or AP-1. Reactions were initiated by the addition of ArfGAP1 (0.1 μM catalytic domain or 0.5 nM full-length ArfGAP1) and terminated by addition of 20 μl of 0.5% SDS followed by 0.5 ml cold charcoal suspension (5% in 50 mM NaH2PO4). After centrifugation, the amount of inorganic [32P]phosphate in the supernatant was determined by scintillation counting and corrected for initial background.
Immunofluorescence
The cDNAs of full-length ArfGAP1 and the catalytic domain ArfGAP1(1–136), both C-terminally fused to a myc-epitope and a His6-tag in pcDNA3.1/myc-His (Invitrogen Life Technologies, Basel, Switzerland) were transfected into COS-1 cells grown on 14-mm glass coverslips using lipofectin (Life Technologies). The cells were fixed with 3% paraformaldehyde for 15 min at room temperature 2 d after transfection, washed in phosphate-buffered saline (PBS), quenched with 50 mM NH4Cl in PBS, and permeabilized with 0.1% Triton X-100 for 10 min. Nonspecific antibody binding was blocked with PBS containing 1% bovine serum albumin. The fixed cells were incubated at room temperature with anti-γ-adaptin (100/3) and rabbit anti-myc antiserum (Abcam, Cambridge, United Kingdom) for 1 h, washed with PBS with albumin, and stained with Alexa488-conjugated goat anti-mouse and Alexa568- conjugated goat anti-rabbit immunoglobulin (Ig) antibodies (Molecular Probes) in PBS with albumin for 30 min. After several washes with PBS with albumin, PBS, and water, the coverslips were mounted in Mowiol 4–88 (Hoechst, Frankfurt, Germany). Staining patterns were analyzed using a Zeiss Axioplan 2 microscope (Oberkochen, Germany) with a KX Series Imaging System (Apogee Instruments, Tucson, AZ).
RESULTS
AP-1 Recruited to Peptidoliposomes Forms High-molecular-weight Complexes in the Absence of Clathrin
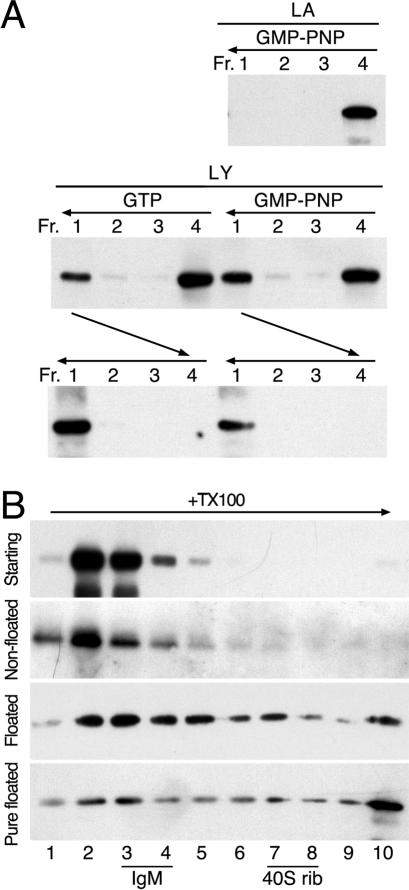
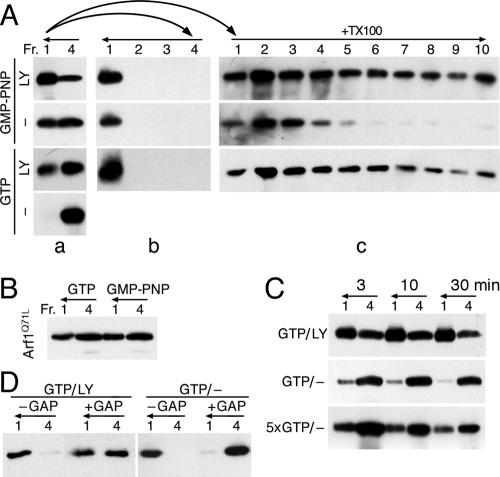
Previously, we have shown that in vitro-purified AP-1 can be recruited to liposomal membranes in the presence of activated Arf1, tyrosine-based signals, and specific lipids (Crottet et al., 2002). To analyze the stability of AP-1 recruitment and the oligomeric state of bound AP-1, peptides corresponding to the wild-type cytoplasmic sequence of Lamp1 (LY) or the tyrosine-to-alanine mutant (LA) were coupled by the lipid reagent MMCC-DPPE to liposomes made of a mixture of soybean lipids previously used for in vitro recruitment assays (e.g., Zhu et al., 1999; Crottet et al., 2002). The resulting peptidoliposomes were incubated at 37°C for 30 min with purified myristoylated Arf1, GTP, or GMP-PNP, and mixed adaptors (containing both AP-1 and AP-2) were isolated from calf brain CCVs. Samples were then supplemented with sucrose to 40% (wt/vol), overlayed with 20% sucrose, and centrifuged for 1 h at 300,000 × g. Four fractions were collected from the top and analyzed by SDS-gel electrophoresis and immunoblotting (Figure 1A). Fraction 1 contained the floated liposomes and any bound proteins, and fraction 4 the initial loading zone. Recruitment of AP-1 to the liposomes required the tyrosine motif and was similar with GTP or GMP-PNP. To assess the stability of AP-1 binding, the floated material of fraction 1 was collected, incubated at 37°C for another 30 min in the absence of nucleotides, and then loaded at the bottom of a new gradient, and centrifuged again as before (Figure 1A, bottom). AP-1 was quantitatively floated with the liposomes to the top of the sucrose cushion, indicating that AP-1 recruited to peptidoliposomes is stably associated with the membrane.
Figure 1.
AP-1 recruited to peptidoliposomes forms high-molecular-weight complexes. (A) Peptidoliposomes made of soybean lipids and presenting LA or LY peptides coupled to MMCC-DPPE were incubated with mixed adaptors, Arf1, and GTP or GMP-PNP. After floatation on a sucrose step gradient, four fractions (Fr. 1–4) were collected from the top and analyzed by immunoblotting for γ-adaptin. Floated liposomes of fraction 1 were further incubated for 30 min at 37°C and floated again as indicated by horizontal arrows. (B) Mixed adaptors were incubated with Arf1, GMP-PNP, and LY peptidoliposomes and centrifuged in a floatation gradient as above. The starting adaptors, the nonfloated fraction 4 and the floated fraction 1 were solubilized with Triton X-100 and centrifuged into a 10–25% sucrose velocity gradient for 5 h at 90,000 × g (horizontal arrow). Ten fractions were collected and analyzed by immunoblotting for γ-adaptin. The floated fraction of a recruitment experiment with purified AP-1 was analyzed in the same way (Pure floated). The positions of the sedimentation markers IgM (19S) and 40S ribosomes are indicated. Individual AP-1 adaptors (∼300 kDa) have a sedimentation coefficient of 7.7S (Nakagawa et al., 2000).
The intrinsic affinity of purified AP-1 to sorting signals is relatively low (Heilker et al., 1996). Stable binding may thus be the result of the additional interactions with membrane-associated Arf1 and lipids and/or due to increased affinity to the tyrosine signal. Alternatively, formation of an oligomer with multiple low-affinity interactions to sorting signals, lipids, and Arf1 might be responsible for the observed stable membrane recruitment. To test the oligomeric state of recruited AP-1, fraction 1 of a floatation experiment using Arf1·GMP-PNP, peptidoliposomes with LY peptides was supplemented with Triton X-100 to solubilize the lipid membrane and was loaded on top of a linear 10–25% sucrose gradient. After centrifugation at 100,000 × g for 5 h, fractions were collected from the top and analyzed by immunoblotting. As controls, the starting adaptor preparation and the nonrecruited material of fraction 4 were analyzed in parallel gradients. AP-1 of these control samples were detected mainly in fraction 2 and 3 of the gradient (Figure 1B). In contrast, recruited AP-1 moved deeply into the gradient and in part even to the bottom fraction. IgM complexes of ∼900 kDa (19 S) and 40 S ribosomes of ∼1400 kDa were found in fractions 3–4 and 7–8 of such a gradient, respectively. Recruited AP-1 was thus present as heterogeneous high-molecular-weight complexes of up to 10 or more units that were resistant to detergent solubilization of the underlying membrane. Similar results were obtained using AP-1 purified to homogeneity (Figure 1B, bottom panel), indicating that only Arf1 and peptidoliposomes are required for AP-1 to oligomerize.
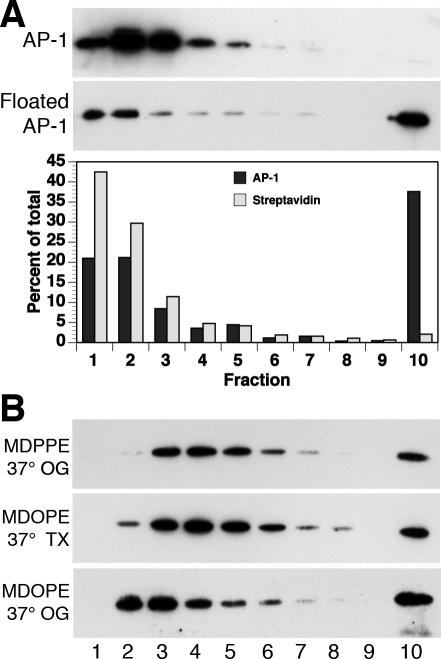
To rule out the possibility that AP-1 may be associated with detergent-insoluble membranes or large mixed micelles, a control experiment was performed by incorporating lipid-(DPPE)-coupled biotin into the peptidoliposomes for the simultaneous recruitment of Arf1/AP-1 and of fluorescently labeled streptavidin. After floatation, the liposome fraction was solubilized and centrifuged into a sucrose gradient as before. Whereas again a large fraction of recruited AP-1 sedimented into the gradient, lipid-anchored streptavidin was recovered almost entirely from the three top fractions (Figure 2A). Binding to saturated lipids thus could not explain sedimentation under the conditions used. We furthermore performed experiments using a lipid reagent with unsaturated oleoyl rather than saturated palmitoyl chains to couple the peptides, and octyl glucoside as a detergent that solubilizes ordered lipid domains more potently than Triton X-100. In addition, detergent was added at 37°C to enhance solubilization. Under all these conditions, a significant fraction of recruited AP-1 sedimented into the second half of the gradient or even the bottom fraction (Figure 2B), excluding insoluble lipid domains as the cause of AP-1 sedimentation.
Figure 2.
AP-1 oligomers are not the result of insoluble membrane domains. (A) Liposomes were made of soybean lipids with LY peptides coupled to MMCC-DPPE and containing biotin-DPPE and mixed with adaptors, Arf1, GMP-PNP, and fluorescently labeled streptavidin. Starting adaptors and the liposome fraction recovered after a flotation gradient were solubilized with Triton X-100 at 4°C and centrifuged into a sucrose velocity gradient as in Figure 1B. Ten fractions were collected and analyzed by immunoblotting for γ-adaptin and by fluorimetry for streptavidin. (B) Soybean liposomes with LY peptides coupled to MMCC-DPPE (MDPPE) or MMCC-DOPE (MDOPE) were incubated with adaptors, Arf1, and GMP-PNP, floated to the top of a first sucrose gradient, solubilized at 37°C with 0.5% Triton X-100 (TX) or octyl glucoside (OG) and centrifuged at room temperature into a 10–25% sucrose velocity gradient containing 0.2% detergent as in Figure 1B. Fractions were analyzed by immunoblotting for γ-adaptin.
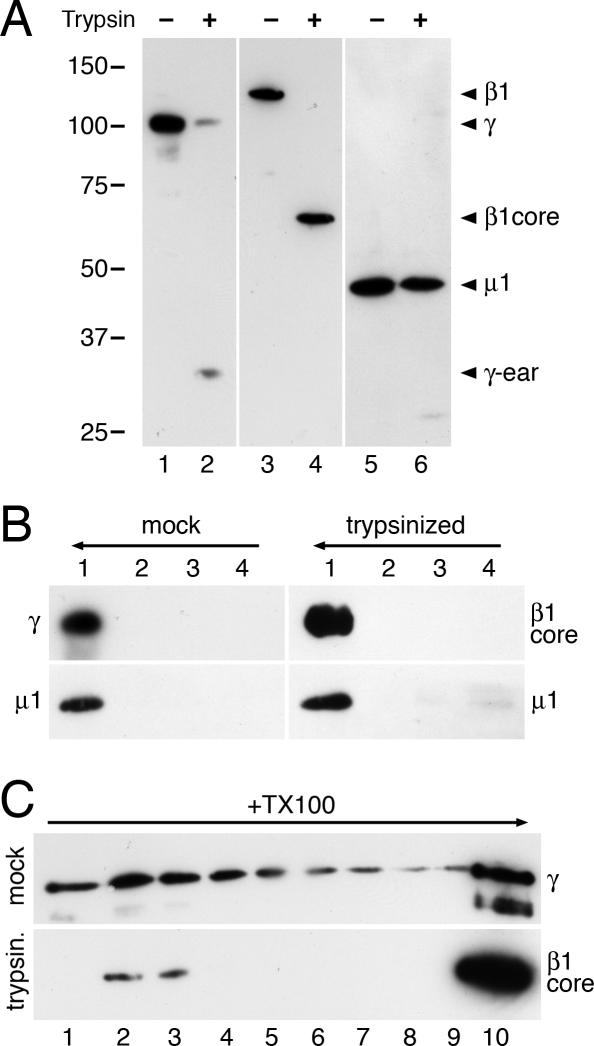
To test whether the C-terminal appendage (ear) domains of γ- or β1-adaptins are involved in forming the oligomers, purified AP-1 adaptors were subjected to limited proteolysis by trypsin. As shown in Figure 3A, the ear domains of the two adaptins were efficiently removed, whereas the μ1 subunit remained largely intact. Both the mock-treated and the trypsinized AP-1 preparations were strongly recruited to peptido-liposomes as shown by immunoblot analysis for μ1 and γ, and μ1 and the β1 core, respectively, after a floatation gradient (Figure 3B). This is consistent with the earlier observation that AP-1 appendages are dispensible for recruitment to Golgi membranes (Traub et al., 1995). On detergent solubilization of the floated liposome fractions, both the intact AP-1 complexes and (even more effectively) the shaved AP-1 core domains sedimented as high-molecular-weight complexes (Figure 3C). This result indicates that the appendage domains of β1 and γ adaptins are not required for oligomer formation.
Figure 3.
Adaptin appendage domains are not required for membrane recruitment and oligomerization. (A) Purified AP-1 adaptors were subjected to limited proteolysis with trypsin or incubated without protease. The products were analyzed by immunoblot analysis using antibodies directed against the hinge segment of γ-adaptin (lanes 1 and 2), against the core domain of β1 adaptin (lanes 3 and 4), or against the μ1 subunit (lanes 5 and 6). The positions of marker proteins are indicated with their molecular weights in kDa. (B) Peptidoliposomes preincubated with Arf1 and GMP-PNP for 30 min at 37°C were mixed on ice with mock-treated or trypsinized AP-1 for 15 min before loading on a floatation gradient as in Figure 1A. Four fractions were collected and analyzed by immunoblotting with the indicated antibodies. (C) The floated fractions 1 of the floatation gradients were solubilized with Triton X-100 and centrifuged into a 10–25% sucrose velocity gradient as in Figure 1B. Fractions were analyzed by immunoblotting for γ-adaptin or the β1 core domain, as indicated. The absence of a 100-kDa band in lane 3 illustrates the absence of β2 and thus of AP-2 complexes.
AP-1 Oligomers Disassemble upon GTP Hydrolysis
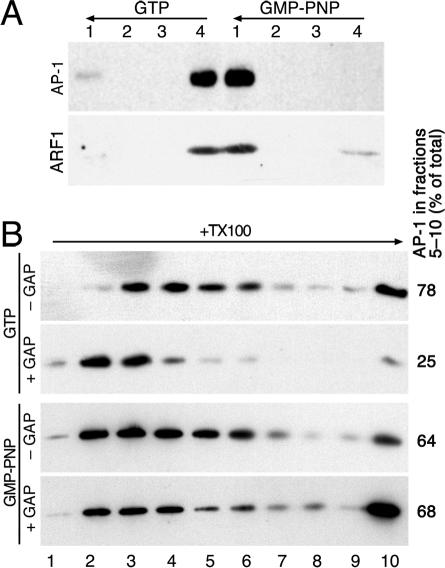
When GTP was used instead of GMP-PNP to recruit AP-1 to LY peptidoliposomes, AP-1 oligomers were similarly observed upon solubilization and sedimentation into a sucrose gradient (Figure 4B, top panel). To analyze the consequences of GTP hydrolysis, we tested the effect of recombinant ArfGAP1 added to membrane-recruited AP-1. ArfGAP1 had been shown to interact with the ear domain of the γ-adaptin subunit of AP-1 and to be present in purified CCVs (Hirst et al., 2003). Floated peptidoliposomes with AP-1 recruited with Arf1 and GTP or GMP-PNP were incubated with ArfGAP1 for 30 min at 37°C. The mixtures were loaded at the bottom of a second floatation gradient and the liposomes were floated again. When GTP had been used to recruit AP-1, the adaptors were efficiently released from the liposomes and recovered in the loading zone (fraction 4; Figure 4A). Arf1 (present in excess of adaptors) was also released, indicating hydrolysis to Arf1·GDP, which dissociates from the membrane. In addition, AP-1 oligomers were disassembled to a large extent upon incubation with ArfGAP1, because AP-1 was detected in fractions 2 and 3 after detergent solubilization and sucrose gradient centrifugation (Figure 4B, upper panels). In the parallel experiment using the non-hydrolyzable nucleotide GMP-PNP, AP-1 remained stably associated with the liposomes (Figure 4A) in an oligomeric state (Figure 4B, lower panels), indicating that ArfGAP1 indeed exerts its effect by inducing GTP hydrolysis in Arf1. These results also indicate that Arf1·GTP is still part of the AP-1 oligomer structures.
Figure 4.
GTP hydrolysis induces dissociation of AP-1 oligomers. LY peptidoliposomes were incubated with adaptors, Arf1, and GTP or GMP-PNP, and floated on a sucrose step gradient. (A) The floated fractions were incubated with ArfGAP1 and subjected to a second floatation. Four fractions were collected and analyzed for γ-adaptin and Arf1. (B) The floated fractions were incubated with or without ArfGAP1, solubilized with Triton X-100 and centrifuged into a velocity sucrose gradient as in Figure 1B. Ten fractions were collected and analyzed by immunoblotting for γ-adaptin. AP-1 recovered in fractions 5–10 of the gradients was determined in percent of the total and listed on the right.
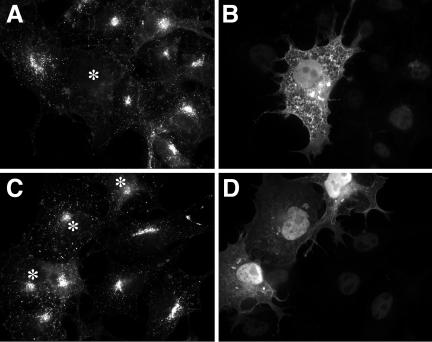
To examine the functional interaction of ArfGAP1 with AP-1 in vivo, we tested the effect of overexpression of ArfGAP1 on AP-1 localization in transfected COS-1 cells. In confirmation of a previous report (Janvier et al., 2003), overexpression of full-length ArfGAP1 strongly reduced Golgi-localized AP-1 (Figure 5, A and B). In addition, AP-1 localization to peripheral structures representing endosomes was also reduced, which argues against an indirect effect of ArfGAP1 on Golgi organization via COPI (Aoe et al., 1997). Overexpression of the catalytic domain of ArfGAP1 (residues 1–136) did not reduce membrane association of AP-1, demonstrating that, as in the COPI system (Huber et al., 1998), the noncatalytic domain of ArfGAP1 is required for membrane targeting and in vivo activity. The effect of ArfGAP1 overexpression on AP-1 localization in vivo together with the previous finding that ArfGAP1 interacts with the γ appendage of AP-1 (Hirst et al., 2003) suggests a functional role for ArfGAP1 in the formation of AP-1/clathrin coats.
Figure 5.
ArfGAP1 overexpression reduces membrane association of AP-1 at the trans-Golgi network and on endosomes. COS-1 cells were transfected with myc-tagged full-size ArfGAP1 (A and B) or catalytic domain ArfGAP1(6–136) (C and D) and stained for AP-1 (anti-γ adaptin; A and C) and for the myc-epitope to visualize transfected cells (B and D). Transfected cells are indicated by asterisks in A and C. AP-1 localization in the perinuclear Golgi area and on endosomes in the cell periphery is strongly reduced in cells expressing full-size ArfGAP1, but not in cells expressing the catalytic domain only.
AP-1 Stimulates the Activity of ArfGAP1
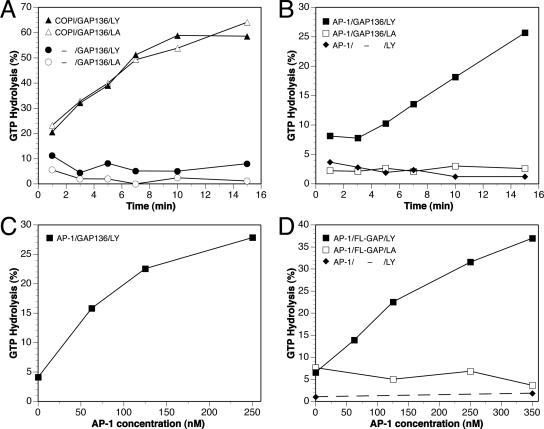
Coatomer and the sorting signal of hp24a have previously been shown to modulate the activity of ArfGAP1 with potential physiological roles in COPI coat formation (Goldberg, 1999; Szafer et al., 2001). We investigated whether AP-1 also influences ArfGAP1 activity. Myristoylated Arf1 was loaded with [γ-32P]GTP on liposomes presenting either the LY or the control LA peptide, and the effect of coat proteins on GAP-dependent GTP hydrolysis was monitored. Because a previous study (Szafer et al., 2000) showed that the recruitment of ArfGAP1 to liposomes through its noncatalytic domain masks the effect of coatomer on GAP activity, we initially used the catalytic fragment of ArfGAP1 in our assays. Although the catalytic fragment alone at a concentration of 0.1 μM did not induce significant GTP hydrolysis (Figure 6A, circles), coatomer strongly stimulated GTP hydrolysis in the presence of either LA or LY liposomes (Figure 6A, triangles). Purified AP-1 also enhanced GAP activity of the catalytic fragment, but in this case, GAP stimulation was only observed with LY peptidoliposomes (Figure 6B, squares). The effect of AP-1 was dose-dependent (Figure 6C). When purified AP-1 was added to LY liposomes in the absence of ArfGAP1, no GTP hydrolysis was observed, excluding a contaminating GAP activity in the adaptor preparation (Figure 6B, diamonds).
Figure 6.
AP-1 stimulates ArfGAP1 activity. Arf1 was activated in the presence of [γ-32P]GTP on liposomes presenting LY (filled symbols) or LA peptides (empty symbols). After incubation with ArfGAP1 and/or effectors, free phosphate released by GTP hydrolysis was measured. The time course of GTP hydrolysis in the presence of 1 μM catalytic domain of ArfGAP1 (GAP136) alone (circles) or in the presence of 0.25 μM COPI coatomer (triangles) is shown in A, and with 0.25 μM purified AP-1 (squares) in B. No hydrolysis was induced by AP-1 without ArfGAP1 (diamonds). The concentration dependence of AP-1 stimulation of the catalytic domain and of full-length ArfGAP1 (FL-GAP; 0.5 nM) is shown in C and D, respectively.
We next investigated whether AP-1 can also stimulate the activity of full-length ArfGAP1. Because in the presence of liposomes full-length ArfGAP1 is much more active than the catalytic fragment (Szafer et al., 2000), the full-length protein was used at a very low concentration of 0.5 nM. Under these conditions, AP-1 stimulated the activity of full-length ArfGAP1 in a dose-dependent manner. As with the catalytic fragment, GAP stimulation depended on the presence of the tyrosine sorting signal (Figure 6D). This signal dependence of ArfGAP1 stimulation by AP-1 is likely to be the result of the recruitment of AP-1 to the membranes, which promotes the interaction of AP-1 with Arf1.
Sorting Signals Are Necessary for Oligomerization of AP-1 in the Presence of Cytosol and Modulate GTP Hydrolysis
To study the role of sorting signals in coat formation, we analyzed the recruitment of AP-1 from cytosol to soybean liposomes. It has been observed that in the presence of cytosol, AP-1 can be bound to liposomes even in the absence of cargo signals (Zhu et al., 1999; Crottet et al., 2002). As shown in Figure 7A, binding of AP-1 recruited from cytosol in the presence of GMP-PNP to liposomes with or without LY peptides was equally stable, because AP-1 remained quantitatively associated with the liposomes during a second floatation (b). This is consistent with the proposal that AP-1 can be bound to a cytosol-derived “docking component(s)” (Zhu et al., 1999). However, solubilization of the membrane and velocity centrifugation (c) revealed that only AP-1 recruited to membranes presenting the functional sorting peptide assembled into oligomers.
Figure 7.
AP-1 recruitment from cytosol. (A) Bovine brain cytosol was supplemented with Arf1, GMP-PNP, or GTP, and liposomes with or without LY peptides as indicated and incubated for 30 min at 37°C. After a first step gradient floatation (a), the floated fraction 1 was either incubated for 30 min at 37°C and subjected to a second floatation (b), or solubilized with Triton X-100 and sedimented into a sucrose gradient (c) as in Figure 1B. (B) Liposomes without sorting peptides were incubated in the presence of GTP or GMP-PNP with cytosol supplemented with hydrolysis-deficient Arf1Q71L before floatation. (C) Liposomes with or without LY peptides were incubated with cytosol, Arf1, and either GTP at the normal concentration of 2 mM (GTP) or at 10 mM (5×GTP). After 3, 10, or 30 min at 37°C, the mixture was chilled on ice and floated on a step gradient. (D) AP-1 recruited from cytosol to liposomes with or without LY peptides in the presence of 10 mM GTP for 10 min and floated on a first gradient were incubated with (+GAP) or without ArfGAP1 (-GAP) and subjected to a second floatation. Five times less cytosol was used with LY peptidoliposomes to have the same amount of AP-1 and lipid in all samples. The indicated fractions were analyzed by immunoblotting for γ-adaptin.
In the presence of 2 mM GTP, AP-1 from cytosol could only be recruited to liposomes presenting LY peptides, where it was found as oligomers (Figure 7A). In the absence of sorting signals, no association of AP-1 with floated liposomes was observed. This suggested that AP-1 recruited via a cytosolic factor was rapidly released by Arf1·GTP hydrolysis stimulated by GAP activity from the cytosol. Indeed, supplementing the GTPase-deficient mutant Arf1Q71L instead of wild-type Arf1 supported stable AP-1 recruitment also in the presence of GTP (Figure 7B). Transient recruitment of AP-1 in the absence of cargo signals could also be directly observed after short incubation times of 3 or 10 min, after which the samples were chilled on ice and analyzed by liposome floatation (Figure 7C, middle row). In contrast, AP-1 binding to liposomes presenting cargo signals is complete after 3 min and is essentially stable (top row). At a constant GTP concentration, a steady-state situation would be expected where Arf1 activation and adaptor recruitment is balanced by hydrolysis and dissociation. The gradual decline of liposome-associated AP-1 could thus be due to the consumption of GTP by Arf1 and by other cytosolic GTPases with time. This was confirmed by the observation of increased and prolonged recruitment of AP-1 when the initial GTP concentration was raised fivefold (bottom row). Under these conditions, floated AP-1 remained liposome-bound in a second floatation (Figure 7D), indicating that the cytosolic GAPs did not remain associated. On incubation of floated liposomes with exogenous ArfGAP1, AP-1 adaptors recruited without peptides were considerably more sensitive to hydrolysis-induced release than those with LY peptides (Figure 7D). These findings indicate that binding of AP-1 to liposomes via putative cytosolic factor(s) is a short-lived stage, timed by GTP hydrolysis and that interaction with sorting signals triggers oligomerization and stabilizes Arf1·GTP by reducing GAP activity.
DISCUSSION
Clathrin coats consist of two layers. Adaptor complexes bind to membrane lipids and activated, membrane-associated G-proteins. In addition, specific docking proteins have been proposed to contribute to site specificity of AP-1 (Zhu et al., 1998, 1999) and AP-2 recruitment (Zhang et al., 1994; Haucke and De Camilli, 1999). Adaptors recognize sorting signals and thus select cargo to be incorporated into the forming vesicles. They recruit clathrin, which polymerizes to form a basket-like coat in a process that is generally believed to laterally collect the membrane-bound adaptors and to concentrate the associated cargo. Here we provide evidence that AP-1 recruited with Arf1·GTP to liposomes presenting cargo signals assembles into high-molecular-weight complexes even in the absence of clathrin. This oligomerization occurs via the adaptor core, because it is independent of the C-terminal appendage domains of β1- and γ-adaptins, including the hinge segments which contain the clathrin-binding sites.
The AP-1 oligomers we have observed might correspond to the AP-1 structures detected in cells where clathrin had been sequestered away by overexpression of auxilin or AP180 (Zhao et al., 2001). Rather than collecting individual adaptors into a coat, clathrin may use oligomers of AP-1 as platforms for its own recruitment and polymerization. Adaptor self-oligomerization may also be the basis for the formation of AP-3 coats, which do not always require clathrin to produce vesicles (Faundez et al., 1998; Shi et al., 1998). Another system where the coat assembles in two layers is the COPII coat. Sar1·GTP and the Sec23/24 dimer first associate with the membrane and in turn recruit the filamentous Sec13/31 coat. Whether Sec23/24 also oligomerizes upon recruitment with Sar1·GTP before association with Sec13/31 remains to be tested.
Although AP-1 could also be recruited to liposomes lacking LY peptides when cytosol was present, AP-1 oligomerization only occurred when sorting signals were present. This is reminiscent of the observation that coatomer and AP-2 oligomerize in solution when exposed to high concentrations of sorting peptides (Reinhard et al., 1999; Haucke and Krauss, 2002). However, AP-1 oligomerization is in addition dependent on Arf1·GTP, because ArfGAP1-induced GTP hydrolysis triggered disassembly of the oligomers and the release of AP-1 from the membrane. This is also reflected in vivo where overexpression of ArfGAP1 strongly reduces membrane association of AP-1 throughout the cell.
One of our main findings is that AP-1 stimulates ArfGAP1-induced GTP hydrolysis on Arf1. The interaction observed in vitro between the appendage domain of γ-adaptin and the C-terminal noncatalytic portion of ArfGAP1 (Hirst et al., 2003) cannot by itself be responsible for GAP stimulation by AP-1, because the catalytic domain of ArfGAP1 (residues 1–136) was also stimulated by AP-1. This suggests the existence of a second AP-1 interaction site residing in the catalytic part of ArfGAP1. A similar two-site interaction of ArfGAP1 with coat has recently been described in the COPI system (Lee et al., 2005), where coatomer also efficiently stimulates the activity of the catalytic fragment of ArfGAP1 (Figure 6A and Goldberg, 1999). Alternatively, binding of AP-1 to Arf1·GTP might render Arf1 more sensitive to ArfGAP1.
GAP stimulation by AP-1 was found to be signal-dependent (Figure 6B). The simplest explanation for this observation is that in the absence of cytosol, sorting signals are required to recruit AP-1 to liposomes and thus bring it into proximity with the membrane-associated Arf1·GTP. Our finding that coatomer stimulates the activity of ArfGAP1 catalytic fragment independently of sorting peptides is in line with the observation that coatomer interacts with soybean liposomes independently of COPI cargo signals (Drake et al., 2000).
Cytosol-mediated recruitment of AP-1 to liposomes lacking sorting signals was stable only in the presence of non-hydrolyzable GTP analogs or a GTPase-deficient Arf1 mutant. In the presence of GTP, recruitment was short-lived, suggesting that the AP-1/Arf1·GTP complex in association with the cytosolic factor is highly susceptible to cytosolic GAPs. By contrast, AP-1 recruited in the presence of GTP and sorting signals was rather stably associated with liposomes, regardless of the presence of cytosol. Even after removal of the bulk of cytosol by floatation of the liposomes, AP-1 was clearly more sensitive to added ArfGAP1 when recruited in the absence of sorting signals (Figure 7D). This suggests a role of sorting signal in the regulation of GTP hydrolysis. Tyrosine-containing signals appear to reduce AP-1-dependent stimulation of ArfGAP1 activity. Such inhibition could be mediated by the interaction of tyrosine signals with AP-1 and/or with ArfGAP1, similar to the previously described inhibitory effect of the p24a cytosolic peptide on ArfGAP1 activity (Goldberg, 2000; Lanoix et al., 2001). In the GAP activity measurements (Figure 6), because signals were required for AP-1 recruitment to the liposome, this inhibited level of GAP stimulation was determined.
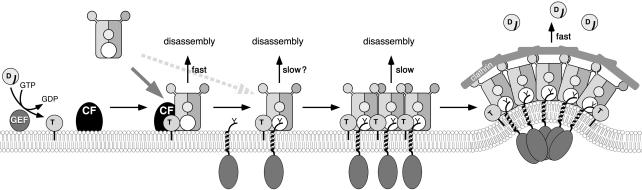
Together these findings fit into a model as illustrated in Figure 8. In vivo, Arf1 is activated by a specific GEF (e.g., BIG2 at the trans-Golgi network; Shinotsuka et al., 2002). Arf1·GTP, appropriate lipids, and putative cytosol-derived factor(s) create binding sites for AP-1. The resulting complex interacts with ArfGAP1 (not drawn in Figure 8 for simplicity) and stimulates its GAP activity, starting the GTPase timer. In the absence of cargo, GTP hydrolysis rapidly leads to dissociation of the complex. On binding of AP-1 to cargo signals, AP-1/Arf1·GTP oligomerizes to larger complexes, probably releasing the docking factor. GTP hydrolysis still triggers disassembly of the oligomers and release of AP-1 from the membrane. However, GAP stimulation by the AP-1/cargo oligomers is weaker, providing more time to complete coat formation, i.e., to recruit and assemble the clathrin layer. It has recently been shown that positive membrane curvature strongly stimulates ArfGAP1 activity by recruiting more GAP to the membrane (Bigay et al., 2003). Membrane deformation by the forming clathrin structure is thus likely to enhance GTP hydrolysis and release of Arf1·GDP.
Figure 8.
A model for AP-1/clathrin coat recruitment and the role of GTP hydrolysis. Gray arrows indicate recruitment of AP-1 to the membrane via Arf1·GTP and either a cytosolic factor (CF) or directly to cargo proteins with tyrosine motifs (Y). On interaction with cargo, AP-1 oligomerizes. GTP hydrolysis induced by ArfGAP1 (not drawn for simplicity) causes AP-1 dissociation unless the clathrin layer has been assembled. Arf1·GTP hydrolysis by ArfGAP1 is differentially stimulated by the AP-1/CF complex, the AP-1/cargo oligomers, and by membrane curvature limiting the time available for the next step in productive coat formation and preparing the adaptor layer for subsequent disassembly, respectively. See also Discussion.
The fact that only traces of Arf1 are detectable in purified CCVs (Zhu et al., 1998) suggests that GTP hydrolysis does not necessarily cause uncoating of the full coat. In our assays using cytosol, we were unable to detect significant amounts of clathrin with the floated liposomes (unpublished data), suggesting that under the conditions used clathrin recruitment and coat completion was not reconstituted. Disassembly of the clathrin layer of CCVs was shown to be catalyzed by hsc70 and its cofactor auxilin or auxilin2/cyclin G-associated kinase (GAK; Ungewickell et al., 1995; Umeda et al., 2000). On clathrin release, the AP-1 layer without active Arf1 is already prepared for dissociation. In addition to this mechanism, it has been shown that phosphorylation of μ1, most likely by GAK, enhances interaction with mannose-6-phosphate receptors, but not with tyrosine motifs as used here, and that dephosphorylation by protein phosphatase 2A stimulates AP-1 release from CCVs in the presence of hsc70 (Ghosh and Kornfeld, 2003). Although GAP activity may not be sufficient for the late stages of CCV disassembly, our results indicate that ArfGAP1 participates in the initial steps of coat formation. Because the rate of GTP hydrolysis is regulated by AP-1 and cargo availability, the Arf1 GTPase and its GAP contribute to cargo recruitment and productive coat formation.
Acknowledgments
We thank Drs. Klaus Fiedler, Tina Junne, Antonius Rolink, and Ernst Ungewickell for the generous gift of reagents and Hans-Peter Hauri for critically reading the manuscript. This work was supported by Grants 31–061579.00 from the Swiss National Science Foundation (M.S.) and 448/04 from Israel Science Foundation (D.C.). B.M. was supported by the Maurice E. Müller Institute at the Biozentrum.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-06-0568) on August 10, 2005.
Abbreviations used: AP, adaptor protein; Arf1, ADP-ribosylation factor 1; CCV, clathrin-coated vesicle; COP, coat protein; GAK, cyclin G-associated kinase; GAP, GTPase-activating protein; GEF, guanine nucleotide exchange factor; GMP-PNP, guanylyl imido-diphosphate; Lamp-1, lysosome-associated membrane protein-1; MMCC-DPPE or -DOPE, N-((4-maleimidylmethyl)cyclohexane-1-carbonyl)-1,2-dipalmitoyl- or -dioleoyl-sn-glycero-3-phosphoethanolamine.
References
- Ahle, S., Mann, A., Eichelsbacher, U., and Ungewickell, E. (1988). Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 7, 919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahle, S., and Ungewickell, E. (1986). Purification and properties of a new clathrin assembly protein. EMBO J. 5, 3143-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny, B., Madden, D., Hamamoto, S., Orci, L., and Schekman, R. (2001). Dynamics of the COPII coat with GTP and stable analogues. Nat. Cell Biol. 3, 531-537. [DOI] [PubMed] [Google Scholar]
- Aoe, T., Cukierman, E., Lee, A., Cassel, D., Peters, P. J., and Hsu, V. W. (1997). The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J. 16, 7305-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor, M., and Traub, L. M. (2002). Cargo selection in vesicular transport: the making and breaking of a coat. Traffic 3, 537-546. [DOI] [PubMed] [Google Scholar]
- Bigay, J., Gounon, P., Robineau, S., and Antonny, B. (2003). Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature 426, 563-566. [DOI] [PubMed] [Google Scholar]
- Bremser, M., Nickel, W., Schweikert, M., Ravazzola, M., Amherdt, M., Hughes, C.A., Sollner, T. H., Rothman, J. E., and Wieland, F. T. (1999). Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell 96, 495-506. [DOI] [PubMed] [Google Scholar]
- Brown, G. E., Kolb, A. J., and Stanley, W. M., Jr. (1974). A general procedure for the preparation of highly active eukaryotic ribosomes and ribosomal subunits. Methods Enzymol. 30, 368-387. [DOI] [PubMed] [Google Scholar]
- Crottet, P., Meyer, D. M., Rohrer, J., and Spiess, M. (2002). ARF1.GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes. Mol. Biol. Cell 13, 3672-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman, E., Huber, I., Rotman, M., and Cassel, D. (1995). The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science 270, 1999-2002. [DOI] [PubMed] [Google Scholar]
- Drake, M. T., Zhu, Y., and Kornfeld, S. (2000). The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes. Mol. Biol. Cell 11, 3723-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez, V., Horng, J. T., and Kelly, R. B. (1998). A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell 93, 423-432. [DOI] [PubMed] [Google Scholar]
- Franco, M., Chardin, P., Chabre, M., and Paris, S. (1995). Myristoylation of ADP-ribosylation factor 1 facilitates nucleotide exchange at physiological Mg2+ levels. J. Biol. Chem. 270, 1337-1341. [DOI] [PubMed] [Google Scholar]
- Ghosh, P., and Kornfeld, S. (2003). AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J. Cell Biol. 160, 699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, J. (1999). Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell 96, 893-902. [DOI] [PubMed] [Google Scholar]
- Goldberg, J. (2000). Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell 100, 671-679. [DOI] [PubMed] [Google Scholar]
- Haucke, V., and De Camilli, P. (1999). AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science 285, 1268-1271. [DOI] [PubMed] [Google Scholar]
- Haucke, V., and Krauss, M. (2002). Tyrosine-based endocytic motifs stimulate oligomerization of AP-2 adaptor complexes. Eur. J. Cell Biol. 81, 647-653. [DOI] [PubMed] [Google Scholar]
- Heilker, R., Manning-Krieg, U., Zuber, J. F., and Spiess, M. (1996). In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting. EMBO J. 15, 2893-2899. [PMC free article] [PubMed] [Google Scholar]
- Hirst, J., Motley, A., Harasaki, K., Peak Chew, S. Y., and Robinson, M. S. (2003). EpsinR: an ENTH Domain-containing Protein that Interacts with AP-1. Mol. Biol. Cell 14, 625-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, I., Cukierman, E., Rotman, M., Aoe, T., Hsu, V. W., and Cassel, D. (1998). Requirement for both the amino-terminal catalytic domain and a noncatalytic domain for in vivo activity of ADP-ribosylation factor GTPase-activating protein. J. Biol. Chem. 273, 24786-24791. [DOI] [PubMed] [Google Scholar]
- Huber, I., Rotman, M., Pick, E., Makler, V., Rothem, L., Cukierman, E., and Cassel, D. (2001). Expression, purification, and properties of ADP-ribosylation factor (ARF) GTPase activating protein-1. Methods Enzymol. 329, 307-316. [DOI] [PubMed] [Google Scholar]
- Janvier, K., Craig, H., Hitchin, D., Madrid, R., Sol-Foulon, N., Renault, L., Cherfils, J., Cassel, D., Benichou, S., and Guatelli, J. (2003). HIV-1 Nef stabilizes the association of adaptor protein complexes with membranes. J. Biol. Chem. 278, 8725-8732. [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T. (2000). Three ways to make a vesicle. Nat. Rev. Mol. Cell. Biol. 1, 187-198. [DOI] [PubMed] [Google Scholar]
- Lanoix, J., Ouwendijk, J., Lin, C. C., Stark, A., Love, H. D., Ostermann, J., and Nilsson, T. (1999). GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO J. 18, 4935-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix, J., Ouwendijk, J., Stark, A., Szafer, E., Cassel, D., Dejgaard, K., Weiss, M., and Nilsson, T. (2001). Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J. Cell Biol. 155, 1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. Y., Yang, J. S., Hong, W., Premont, R. T., and Hsu, V. W. (2005). ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J. Cell Biol. 168, 281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. M., Poon, P. P., Singer, R. A., Johnston, G. C., and Spang, A. (2004). The ArfGAP Glo3 is required for the generation of COPI vesicles. Mol. Biol. Cell 15, 4064-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, J. O., and Kornfeld, S. (1997). Comparative activity of ADP-ribosylation factor family members in the early steps of coated vesicle formation on rat liver Golgi membranes. J. Biol. Chem. 272, 4141-4148. [DOI] [PubMed] [Google Scholar]
- Malsam, J., Gommel, D., Wieland, F. T., and Nickel, W. (1999). A role for ADP ribosylation factor in the control of cargo uptake during COPI-coated vesicle biogenesis. FEBS Lett. 462, 267-272. [DOI] [PubMed] [Google Scholar]
- Matsuoka, K., Morimitsu, Y., Uchida, K., and Schekman, R. (1998a). Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Mol. Cell 2, 703-708. [DOI] [PubMed] [Google Scholar]
- Matsuoka, K., Orci, L., Amherdt, M., Bednarek, S. Y., Hamamoto, S., Schekman, R., and Yeung, T. (1998b). COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93, 263-275. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T., Setou, M., Seog, D., Ogasawara, K., Dohmae, N., Takio, K., and Hirokawa, N. (2000). A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell 103, 569-581. [DOI] [PubMed] [Google Scholar]
- Nickel, W., Malsam, J., Gorgas, K., Ravazzola, M., Jenne, N., Helms, J. B., and Wieland, F. T. (1998). Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPgammaS in vitro. J. Cell Sci. 111(Pt 20), 3081-3090. [DOI] [PubMed] [Google Scholar]
- Paleotti, O., Macia, E., Luton, F., Klein, S., Partisani, M., Chardin, P., Kirchhausen, T., and Franco, M. (2005). The small G-protein Arf6GTP recruits the AP-2 adaptor complex to membranes. J. Biol. Chem. 280, 21661-21666. [DOI] [PubMed] [Google Scholar]
- Pavel, J., Harter, C., and Wieland, F. T. (1998). Reversible dissociation of coatomer: functional characterization of a beta/delta-coat protein subcomplex. Proc. Natl. Acad. Sci. USA 95, 2140-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperkok, R., Whitney, J. A., Gomez, M., and Kreis, T. E. (2000). COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo. J. Cell Sci. 113, 135-144. [DOI] [PubMed] [Google Scholar]
- Poon, P. P., Cassel, D., Spang, A., Rotman, M., Pick, E., Singer, R. A., and Johnston, G. C. (1999). Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 18, 555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo, P. A., and Hirsch, D. S. (2004). Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal. 16, 401-413. [DOI] [PubMed] [Google Scholar]
- Reinhard, C., Harter, C., Bremser, M., Brugger, B., Sohn, K., Helms, J. B., and Wieland, F. (1999). Receptor-induced polymerization of coatomer. Proc. Natl. Acad. Sci. USA 96, 1224-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, M. S., and Bonifacino, J. S. (2001). Adaptor-related proteins. Curr. Opin. Cell Biol. 13, 444-453. [DOI] [PubMed] [Google Scholar]
- Schröder, S., and Ungewickell, E. (1991). Subunit interaction and function of clathrin-coated vesicle adaptors from the Golgi and the plasma membrane. J. Biol. Chem. 266, 7910-7918. [PubMed] [Google Scholar]
- Shi, G., Faundez, V., Roos, J., Dell'Angelica, E. C., and Kelly, R. B. (1998). Neuroendocrine synaptic vesicles are formed in vitro by both clathrin-dependent and clathrin-independent pathways. J. Cell Biol. 143, 947-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinotsuka, C., Yoshida, Y., Kawamoto, K., Takatsu, H., and Nakayama, K. (2002). Overexpression of an ADP-ribosylation factor-guanine nucleotide exchange factor, BIG2, uncouples brefeldin A-induced adaptor protein-1 coat dissociation and membrane tubulation. J. Biol. Chem. 277, 9468-9473. [DOI] [PubMed] [Google Scholar]
- Spang, A., Matsuoka, K., Hamamoto, S., Schekman, R., and Orci, L. (1998). Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc. Natl. Acad. Sci. USA 95, 11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafer, E., Pick, E., Rotman, M., Zuck, S., Huber, I., and Cassel, D. (2000). Role of coatomer and phospholipids in GTPase-activating protein-dependent hydrolysis of GTP by ADP-ribosylation factor-1. J. Biol. Chem. 275, 23615-23619. [DOI] [PubMed] [Google Scholar]
- Szafer, E., Rotman, M., and Cassel, D. (2001). Regulation of GTP hydrolysis on ADP-ribosylation factor-1 at the Golgi membrane. J. Biol. Chem. 276, 47834-47839. [DOI] [PubMed] [Google Scholar]
- Tanigawa, G., Orci, L., Amherdt, M., Ravazzola, M., Helms, J. B., and Rothman, J. E. (1993). Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J. Cell Biol. 123, 1365-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub, L. M., Kornfeld, S., and Ungewickell, E. (1995). Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J. Biol. Chem. 270, 4933-4942. [DOI] [PubMed] [Google Scholar]
- Umeda, A., Meyerholz, A., and Ungewickell, E. (2000). Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur. J. Cell Biol. 79, 336-342. [DOI] [PubMed] [Google Scholar]
- Ungewickell, E., Ungewickell, H., Holstein, S. E., Lindner, R., Prasad, K., Barouch, W., Martin, B., Greene, L. E., and Eisenberg, E. (1995). Role of auxilin in uncoating clathrin-coated vesicles. Nature 378, 632-635. [DOI] [PubMed] [Google Scholar]
- Watson, P. J., Frigerio, G., Collins, B. M., Duden, R., and Owen, D. J. (2004). gamma-COP appendage domain—structure and function. Traffic 5, 79-88. [DOI] [PubMed] [Google Scholar]
- Weiss, M., and Nilsson, T. (2003). A kinetic proof-reading mechanism for protein sorting. Traffic 4, 65-73. [DOI] [PubMed] [Google Scholar]
- Yang, J. S., Lee, S. Y., Gao, M., Bourgoin, S., Randazzo, P. A., Premont, R. T., and Hsu, V. W. (2002). ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J. Cell Biol. 159, 69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. Z., Davletov, B. A., Sudhof, T. C., and Anderson, R. G. (1994). Synaptotagmin I is a high affinity receptor for clathrin AP-2, implications for membrane recycling. Cell 78, 751-760. [DOI] [PubMed] [Google Scholar]
- Zhao, X., Greener, T., Al-Hasani, H., Cushman, S. W., Eisenberg, E., and Greene, L. E. (2001). Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J. Cell Sci. 114, 353-365. [DOI] [PubMed] [Google Scholar]
- Zhu, Y., Drake, M. T., and Kornfeld, S. (1999). ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc. Natl. Acad. Sci. USA 96, 5013-5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., Traub, L. M., and Kornfeld, S. (1998). ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol. Biol. Cell 9, 1323-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]