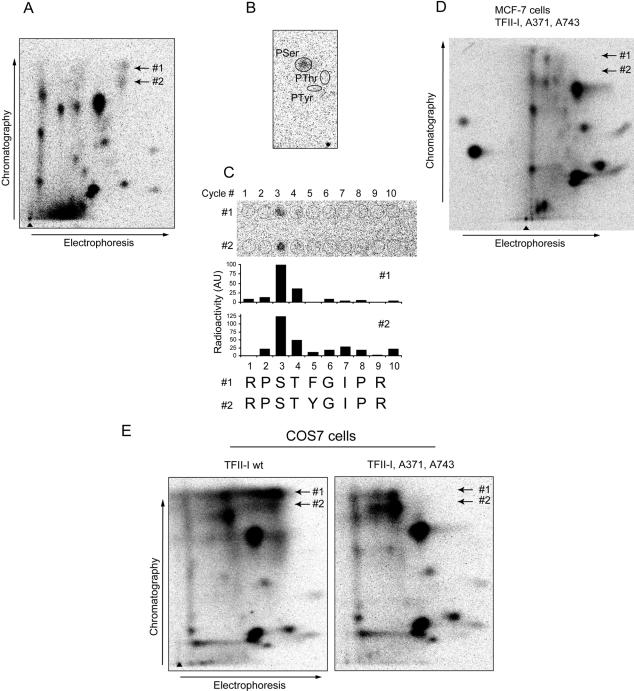
Figure 5.
TFII-I is phosphorylated at serine residues 371 and 743 upon treatment of cells with TGFβ1. (A) Two TGFβ1-induced phosphopeptides, #1 and #2, were identified in a phosphopeptide map of transfected myc-TFII-I that was immunoprecipitated from MCF7-vector cells metabolically labeled with [32P]orthophosphate. Cells were treated with TGFβ1 (5 ng/ml) for 4 h. Migration positions of the peptides are shown by arrows. (B) The phosphopeptides shown in A were eluted and subjected to phosphoamino acid analysis. For both peptides, radioactivity was comigrating with phospho-serine (pSer), and no radioactivity was comigrating with phospho-threonine (pThr) or phospho-tyrosine (pTyr). Migration positions of pSer, pThr, and pTyr are indicated. (C) Radioactivities in both TGFβ1-dependent phosphopeptides were released in the third cycle of radiochemical sequencing (C; top). Localizations of applied fractions are shown by open cycles, and number of cycles is shown (top). Quantification of the signals presented (top of panels) is shown (middle). Cycles of radiochemical degradation are aligned with sequences of two candidate peptides (C; bottom). (D) Phosphopeptide mapping of myc-TFII-I with serine residues 371 and 743 replaced by alanine residues did not show phosphopeptides #1 and #2. TFII-I mutant Ser371,743Ala was transfected in MCF-7 cells, and metabolic labeling, immunoprecipitation, and phosphopeptide mapping were performed as for wild-type myc-TFII-I. Expected migration positions for phosphopeptides #1 and #2 are shown by arrows. (E) Mutation of serine residues 371 and 743 abrogated appearance of two phosphopeptides migrating at identical positions as TGFβ1-dependent phosphopeptides, in myc-TFII-I expressed in COS7 cells. Cells transfected with wild-type or mutant myc-TFII-I were metabolically labeled with [32P]orthophosphate and were incubated with 8-Br-cGMP to activate cotransfected PKG. myc-TFII-I was immunoprecipitated with anti-tag antibodies, and phosphopeptide mapping was performed. Migration positions of phosphopeptides #1 and #2 are shown by arrows.