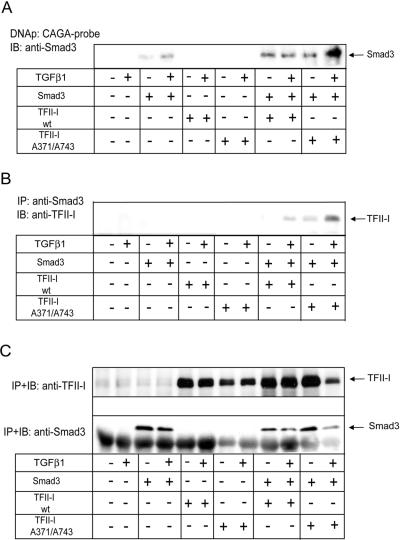
Figure 8.
TFII-I transfection increased binding of Smad3 to the CAGA elements. (A) DNA precipitation assay with immobilized CAGA-probe showed increased binding of myc-Smad3 (Smad3) to the probe upon cotransfection of myc-TFII-I Ser371,743Ala mutant (TFII-I A371/A743) and treatment with TGFβ1, compared with binding of myc-Smad3 cotransfected with wild-type myc-TFII-I (TFII-I wt). COS7 cells were transfected with myc-Smad3 and TFII-I plasmids, and treated with TGFβ1 (5 ng/ml), as indicated. Proteins bound to CAGA probe were separated by SDS-PAGE, and transferred onto nitrocellulose membrane. Smad3 was detected by immunoblotting with anti-Smad3 antibody. The migration position of Smad3 is shown by an arrow. (B) Smad3 and TFII-I form a complex. COS7 cells were transfected with myc-Smad3, myc-TFII-I wild-type and Ser371/743Ala mutant, and empty vector, as described in B, and were treated with TGFβ1 (5 ng/ml), as indicated. Proteins immunoprecipitated with anti-Smad3 antibodies were immunoblotted with anti-TFII-I antibodies. The anti-Smad3 and anti-TFII-I antibodies used recognize also endogenous proteins. Migration position of TFII-I is shown by an arrow. (C) Expression of Smad3 and TFII-I in cells which were used in DNA-precipitation and coimmunoprecipitation assays. Transfection of Smad3 and TFII-I plasmids was performed in identical manners for the two assays, and transfected cells were divided and used for assays at the same time. Expression of Smad3 and TFII-I proteins was evaluated by immunoprecipitation and immunoblotting of Smad3 and TFII-I with respective specific antibodies which recognize epitopes also in endogenous proteins. Migration positions of Smad3 and TFII-I are shown by arrows. Representative experiments out of three (A–C) performed are shown.