Fig. 2. Long MLCK is found in intestinal epithelium and is primarily responsible for Na+-glucose cotransport-induced tight junction regulation.
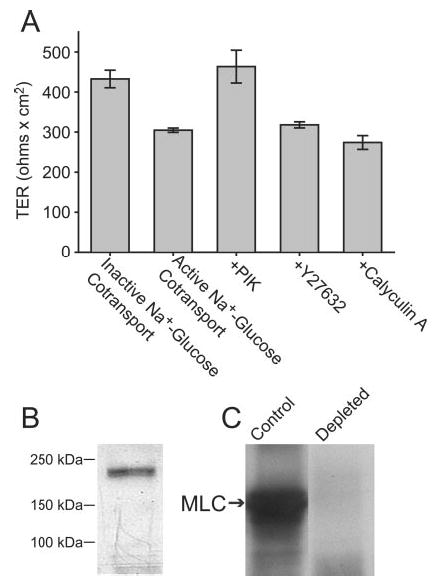
A, Caco-2 monolayers were incubated in Hank’s balanced salt solution with 25 mm glucose (active Na+-glucose cotransport) or 5 mm glucose, 20 mm mannitol, and 2 mm phloridzin (inactive Na+-glucose cotransport), as in Fig. 1, or with 25 mm glucose and the MLCK inhibitor PIK (250 μm), the Rho kinase inhibitor Y27632 (10 μm), or the phosphatase 1 and 2A inhibitor calyculin A (10 nm). TER after 2 h, at which time TER had stabilized, is shown. Activation of Na+-glucose cotransport reduced TER by 30 ± 4%. The addition of PIK elevated TER to that of monolayers with inactive Na+-glucose cotransport. Y27632 and calyculin A had no effect on TER, indicating that MLCK is primarily responsible for Na+-glucose cotransport-dependent tight junction regulation. B, analysis of Caco-2 cell lysates by SDS-PAGE and immunoblot with broadly reactive anti-MLCK monoclonal antibody detects a single band of ~215 kDa. C, immunodepletion of Caco-2 lysates using the same anti-MLCK monoclonal antibody removed 97 ± 4% of MLC kinase activity.
