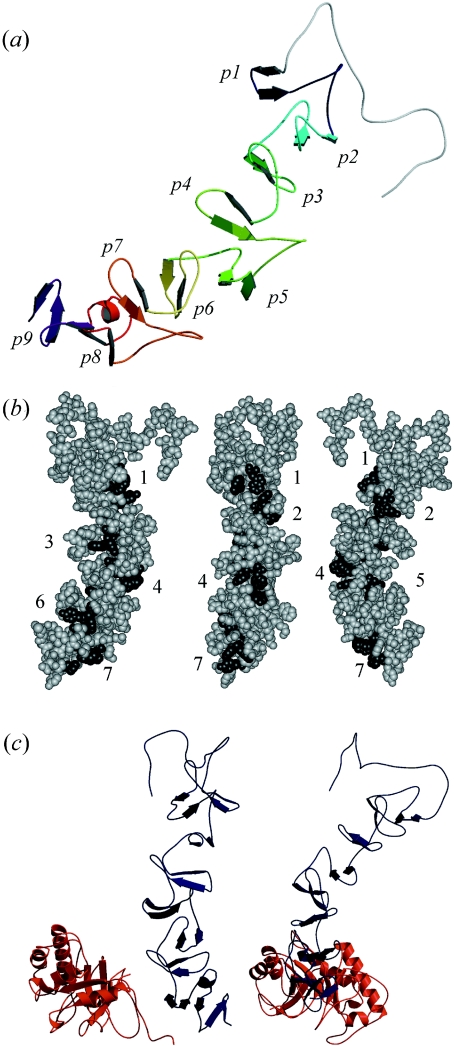
Figure 3. Structural models of the CBM of LytC and the whole enzyme.
(a) The CBM folds in a β-solenoid structure. β-Hairpins of repeating units appear differently coloured, from dark blue (p1) to purple (p9). (b) Disposition of the potential choline-binding sites. Numbers indicate the order of the hydrophobic cavities (dark grey) from the N- to the C-terminal end starting at the p2 repeat. Sites 2 and 5 are located between a short and a large repeat. (c) Different views of the model generated for the whole structure of LytC. The region comprising strands β6–β8 of the CM (red) interacts with repeats p7–p8 of the CBM (dark blue). The C-terminal side of the barrel, where the catalytic cavity is located, points towards the bottom of the Figure.