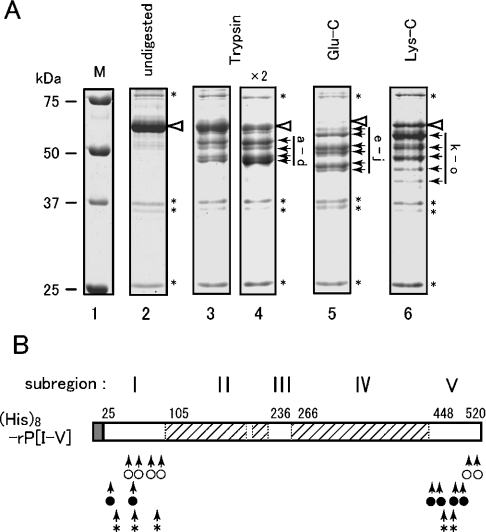
Figure 3. Partial proteolysis of the yeast Ire1 luminal domain.
(A) As described in the Experimental section, His8–rP[I-V] was incubated with trypsin, or endoproteinase Glu-C or Lys-C, and fractionated by SDS/12% PAGE. The gel was then stained with Coomassie Blue and photographed. All lanes are from the same gel. Positions of undigested His8–rP[I-V] and unidentified proteins are respectively marked by white arrowheads and asterisks (*). The major fragments that appear in Table 1 are marked by black arrows and are labelled alphabetically. Lane 1, molecular-mass markers [unstained Precision Plus Protein Standards (Bio-Rad)] (sizes are indicated in kDa); lane 2, undigested; lane 3, trypsin (15 ng); lane 4, trypsin (30 ng); lane 5, Glu-C (240 ng); lane 6, Lys-C (120 ng). (B) Proteolytic cleavage sites in His8–rP[I-V] are represented schematically by arrows for trypsin (○), Glu-C (●) and Lys-C (*) respectively.