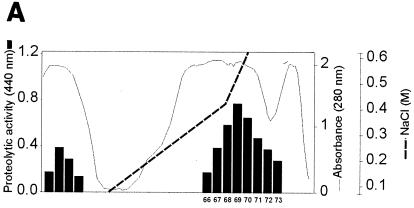
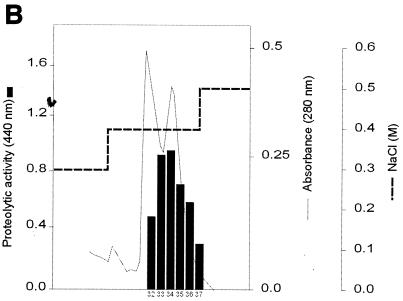
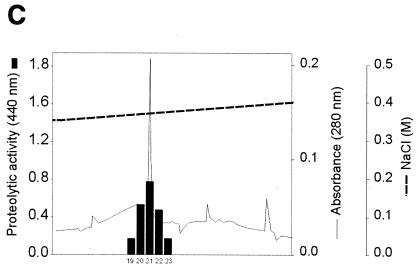
FIG. 3.
Purification of protease II from concentrated culture supernatant of X. nematophila. (A) Separation of two protease fractions by anion-exchange chromatography. Elution consisted of a gradient with 10 mM cacodylate, pH 7.6, containing 0 to 1 M NaCl over 60 min. The protease activity of each fraction, as indicated by the absorbance at 440 nm, was determined under the conditions described in Materials and Methods. (B) Elution of protease II from the HiTrap Q column. The proteins were eluted in a step gradient with 10 mM cacodylate, pH 7.6, containing 0 to 0.5 M NaCl. (C) Elution profile of protease II from a Mono Q column. The proteins were eluted in a linear gradient with 10 mM cacodylate (pH 7.6) containing a 0.35 to 0.45 M concentration of NaCl over 120 min.