Abstract
Unsaturated nitriles are significant in prebiotic and astrochemistry. Dicyanoacetylene, in particular, is a possible precursor of uracil and was previously detected in Titan’s atmosphere. Its null dipole moment hindered detection through rotational spectroscopy in interstellar clouds, and it escaped identification until recently, when its protonated form NC4NH+ was finally detected toward the Taurus molecular cloud (TMC-1) (Agúndez et al., Astronom. Astrophys. 2023, 669, L1). Given the low-temperature conditions of both Titan and TMC-1, a facile formation route must be available. Low-temperature kinetics experiments and theoretical characterization of the entrance channel demonstrated that the CN + HC3N reaction is a compelling candidate for NC4N formation in cold clouds. Here, we report on a combined crossed-molecular beams (CMB) and theoretical study of the reaction mechanism up to product formation, demonstrating that NC4N + H is the sole open channel from low to high temperatures (collision energies). Indeed, unlike other CN reactions, the formation of the isocyano isomer (3-isocyano-2-propynenitrile) was not seen to occur at the high collision energy (44.8 kJ/mol) of the CMB experiment. Preliminary calculations on the related CN + HC5N reaction indicate that the reaction channel leading to NC6N + H is exothermic and occurs via submerged transition states. We therefore expect it to be fast and that the mechanism is generalizable to the entire family of CN +cyanopolyyne reactions. Furthermore, we derive some properties of the related reactions C2H + CNCN (isocyanogen) and CN + HCCNC (isocyanoacetylene): the C2H + CNCN reaction leads to the formation of HC3N + CN, and the main channel of the CN + HCCNC reaction also leads to CN + HC3N. This last reaction efficiently converts isocyanoacetylene and, by extension, any isocyanopolyyne into their cyano counterparts without a net loss of cyano radicals. Finally, we also characterized the entrance channel of the reaction C2H + NC4N and verified that the addition of C2H to all possible sites of NC4N is characterized by a significant entrance barrier, thus confirming that, once formed, dicyanoacetylene terminates the growth of cyanopolyynes via the sequence of steps involving polyynes, cyanopolyynes, and C2H/CN radicals.
Keywords: dicyanoacetylene;, cyanopolyynes;, crossed-molecular beams;, cyano radicals


1. Introduction
Approximately 15–20% of all species identified in the interstellar medium (ISM) are nitriles, a family of N-bearing organic compounds characterized by a cyano (CN) group. Unsaturated nitriles (including vinylcyanoacetylene, cyanoacetyleneallene or aromatic cyanonaphthalene, cyanoacenaphthylene, cyanopyrene, and cyanocoronene) rank among the most complex individual interstellar organic molecules identified to date. − The detection of these complex nitriles was accomplished toward a cold molecular cloud, the Taurus Molecular Cloud (TMC-1), which seems to be particularly rich in nitriles. Furthermore, nitriles have been detected on Titan − as well as in star-forming regions (high-mass and low-mass), − protoplanetary disks, − circumstellar envelopes (CSE) of asymptotic giant branch (AGB) stars − in photodissociation regions (PDR), , and cometary comae. − Their presence in exoplanets has also been speculated. Given their significant prebiotic potential and the conjectured role they may have played in the chemistry that led to the emergence of life, , nitriles have garnered considerable attention, prompting extensive investigation into their formation pathways and astrochemical/photochemical models (see, for instance, refs. , , − ).
More recently, given the increased sensitivity of radio telescopes, the first detection of interstellar dinitriles (molecules containing two cyano groups) has become accessible. The first dinitriles identified were maleonitrile (Z-but-2-enedinitrile or cis-1,2-dicyanoethylene) and malononitrile (propanedinitrile or dicyanomethane), both identified toward TMC-1. Prior to this, isocyanogen (CNCN) and protonated cyanogen had been detected, , while attempts to detect larger saturated dinitriles , and dicyanobenzene failed. Furthermore, and of relevance to the present work, the detection of dicyanoacetylene (2-butynedinitrile; NCCCCN, henceforth indicated by NC4N) in its protonated form NC4NH+ (an otherwise nonobservable apolar dinitrile) finally confirmed its presence, which, together with that of larger dicyanopolyynes, had been postulated long ago. , NC4N is considered a precursor of uracil via its hydrolysis to acetylenedicarboxylic acid and subsequent reaction with urea. Furthermore, in a recent study, a new α-cytidine derivative was successfully synthesized from the reaction of ribose aminooxazoline and dicyanoacetylene. Therefore, it is particularly interesting to understand the formation routes of dicyanoacetylene in space and its possible connection with the emergence of life on Earth.
Both cyanogen (C2N2) and dicyanoacetylene (in the form of ice) had already been identified in the atmosphere of Titan, the massive moon of Saturn. ,, Their detection prompted speculation on their possible formation routes long ago. In the case of dicyanoacetylene, the first suggested formation route was the reaction HCCN + HCCN → NCCCCN + H2, but it was later speculated that HCCN (formed by the reaction N(2D) + C2H2) reacts rather with H or CH3 radicals. The other NC4N formation route suggested by Yung was the reaction
| 1 |
in analogy with the related reaction
| 2 |
A rate coefficient of 10–11 cm3·s–1 was adopted by Yung in his upgraded photochemical model of the atmosphere of Titan, following the indications of experimental measurements by Halpern et al., who later published their work reporting a value of (1.7 ± 0.08) × 10–11 cm3 s–1 at room temperature. A barrier of 1.5 kcal mol–1 (6.3 kJ mol–1) was suggested upon comparison with the rate coefficients of other CN reactions with unsaturated hydrocarbons. This room-temperature data were used by Faure et al. to estimate, using a semiempirical model coupled to the capture theory, the k(T) value in the temperature range from 10 to 295 K. Finally, recent kinetic measurements by using the CRESU technique derived the k(T) below room temperature, reaching a value as low as 22 K. The reaction rate coefficient was seen to increase with decreasing temperature, which is incompatible with the significant entrance barrier inferred by Halpern et al. However, the rate coefficients at low temperatures are smaller than those predicted by Faure et al. In addition to the experimental values, Cheikh Sid Ely et al. also characterized the entrance channel of the potential energy surface (PES) of reaction (1) and were able to reproduce the trend of the experimental rate coefficient as a function of temperature by employing a two-transition-state (2TS) model. A similar trend was also obtained by Valença Ferreira de Aragão et al., who used a semiempirical analysis of the long-range interactions based on an improved Lennard–Jones potential. Both theoretical studies focused only on the entrance channel, and the full PES up to the products was not derived. In addition, both the kinetic experiments by Cheikh Sid Ely et al. and Halpern et al. followed only the decay rate of the reactants and, therefore, could not provide information on the reaction products.
Some information on the global PES of reaction (1) can be derived from the work of Petrie and Osamura, which focuses on the reaction between C3N and HNC as the main formation pathway of NC4N in the atmosphere of Titan. The products of that reaction include, among others, cyanoacetylene and cyano radicals in one channel and dicyanoacetylene and atomic hydrogen in another channel. In the PES by Petrie and Osamura, there is a pathway connecting these products, featuring one intermediate and a transition state. However, the full potential energy surface of the CN + HC3N reaction has yet to be reported, and the CN addition on the N-side has never been considered before. This last aspect could be relevant for high-energy environments since, in the case of the related CN reactions with acetylene and ethylene, crossed-molecular beam (CMB) experiments provided evidence that the channels leading to the isocyano isomeric products (isocyanoacetylene and isocyanoethylene) become open at high collision energies. −
In conclusion, although dicyanoacetylene + H has always been considered the only open reaction channel, a comprehensive study of the PES of the title reaction is still lacking, as is experimental evidence of the nature of the reaction products. In this work, we report the results of a CMB experiment that demonstrates that dicyanoacetylene + H is the sole open reaction channel up to high collision energies (and therefore high temperatures). The CMB results are complemented by the derivation of the complete reactive potential energy surface, in which the CN addition on the N-side is also considered, to verify whether 3-isocyano-2-propynenitrile (NCCCNC, henceforth indicated by the simplified formula CNC3N) can be formed at high collision energies. The formation of isocyano isomers could be relevant in high-temperature environments, such as flames or the CSE of AGB stars and photodissociation regions, where isonitrile species have been identified. ,,
2. Methods
2.1. The Crossed-Molecular Beam Technique with Mass Spectrometric Detection
The crossed-molecular beam (CMB) apparatus, equipped with universal quadrupole mass spectrometric (QMS) detection and time-of-flight (TOF) analysis, used in the present study, has been described in detail elsewhere. , Therefore, only a concise description is provided here. Two collimated, continuous supersonic beams of the reactants are crossed at 90° in a large scattering chamber maintained at 10–5 Pa to ensure single-collision conditions. Reactants and products were detected by a triply differentially pumped ultrahigh-vacuum detection system, maintained at a pressure of less than 10–9 Pa during operation and equipped with an electron impact ionizer, a quadrupole mass filter, and a Daly detector. The entire detector unit can be rotated in the collision plane around an axis that passes through the collision center.
The product angular distribution, N(Θ), that is, the intensity of the products as a function of the laboratory (LAB) scattering angle Θ, was obtained by taking at least five scans per angle, with a counting time of 100 s and by modulating the cyanoacetylene beam with a tuning fork chopper at a frequency of 160 Hz for background subtraction. Velocity distributions of the reactants were determined by measuring in-axis single-shot time-of-flight (TOF) spectra. Product TOF distributions, N(Θ,t), were obtained at selected LAB angles by using the TOF pseudorandom chopping technique, with the pseudorandom wheel (containing four identical sequences of 127 open/closed elements) spinning in front of the entrance of the detector at 328.1 Hz (corresponding to a dwell time of 6 μs/channel).
The beam of CN radicals was generated by the high-pressure radio frequency (RF) discharge beam source successfully used in our laboratory over a number of years to generate intense supersonic beams of atoms and radicals. − More specifically, the CN beam was generated by expanding a gas mixture with the composition CO2(0.8%)/N2(2.5%)/He at 90 hPa (quartz nozzle diameter was 0.45 μm, and RF power 300 W); the CN beam had a peak velocity of 2184 m/s and a speed ratio of 5.5. Typically, in CMB experiments utilizing supersonic beams, the internal energy content of the reactants is minimal due to the extensive cooling of internal degrees of freedom during expansion. Nevertheless, in this case, the beam of CN radicals is formed chemically in situ by the transient species produced in the discharge plasma starting from CO2/N2/He and the radicals maintain a large internal energy content. By using a laser-induced fluorescence (LIF) characterization, we verified that the cooling of the internal excitation is incomplete and that the CN radicals are ro-vibrationally excited. More specifically, the CN vibrational distribution is consistent with a vibrational temperature of 6500 K, while the rotational distribution is bimodal, with two peaks around N = 6 and N = 39–44.
Cyanoacetylene (HC3N) was synthesized by following the two-stage procedure described by Miller and Lemmon (see also Liang et al.). , The HC3N white crystals were stored in a glass vial immersed in a 261 K constant-temperature bath to prevent polymerization and maintain a stable equilibrium vapor pressure. The HC3N beam was produced by expanding the vapor (pressure around 64 hPa) through a 0.1 mm stainless steel nozzle. The internal energy content of the HC3N molecule is expected to be small because the internal degrees of freedom are cooled during the supersonic expansion. The HC3N beam had a peak velocity of 657 m/s and a speed ratio of 3.5.
Under these experimental conditions, the average collision energy (E c ) was 44.8 kJ/mol. The center-of-mass (CM) position angle, ΘCM, was 30.6°.
The scattering measurements were carried out in the LAB system of coordinates, while for the physical interpretation of the scattering process, it is necessary to transform the data (angular, N(Θ), and time-of-flight N(Θ, t) distributions) to a coordinate frame of reference that moves with the center-of-mass (CM) of the colliding system. The relation between the LAB and CM product flux is given by I LAB (Θ,v) = I CM (θ,u) × v 2/u 2, where Θ and v are the LAB scattering angle and velocity, respectively, while θ and u are the corresponding CM quantities (see the velocity vector, or “Newton,” diagram in Figure ). Since the mass spectrometric detector measures the number density of products, N(Θ), rather than the flux, the actual relation between the LAB density and the CM flux is given by N LAB (Θ,v) = I CM (θ,u) × v/u 2. The final outcome of a reactive scattering experiment is therefore the so-called differential cross-section I CM (θ,u), which is commonly factorized into the product of the velocity (or translational energy) distribution, P(u) (or P(E’ T )), and the angular distribution, T(θ). Because of the finite resolution of experimental conditions, i.e., finite angular and velocity spread of the reactant beams and angular resolution of the detector, the LAB-CM transformation is not single-valued, and analysis of the laboratory data is carried out by the usual forward convolution procedure in which trial CM angular, T(θ), and translational energy, P(E’ T ), distributions are assumed, averaged, and transformed to the LAB for comparison with the experimental data until the best fit of the LAB distributions is achieved. The best-fit CM T(θ) and P(E’ T ) functions contain all of the information about the reaction dynamics.
1.

Laboratory angular distribution of the NC4N product at m/z = 76 from the reaction CN (X2Σ+) + HC3N at E c = 44.8 kJ/mol using a 70 eV electron energy at an emission current of 2 mA and the corresponding velocity vector (Newton) diagram. The dots represent the experimental data (an average of at least 5 scans) and their standard deviation. The solid black line in the upper figure represents the calculated distribution using the best-fit CM angular and translational energy functions from Figure . ΘCM defines the CM position angle. The circle in the Newton diagram delimits the maximum center-of-mass speed and therefore, the angular range within which the NC4N product can be scattered (see text). The analogous circle for CNC3N could not be drawn because it is too small.
2.2. Electronic Structure Calculations of the Reaction Potential Energy Surface
The potential energy surface of the doublet CN–HC3N system was investigated by searching for and optimizing the relevant stationary points and product channels. Calculations were performed adopting an unrestricted formalism using the Gaussian 09 code. Following a well-established computational scheme, the geometries of minima and saddle points were optimized using a less expensive method compared to the one employed to get more accurate energy values. ,,− Preliminary benchmark calculations to test the theoretical methods were presented in ref. .
Geometry optimization calculations were performed using density functional theory (DFT), with the Becke-3-parameter exchange and Lee–Yang–Parr correlation (B3LYP) , in conjunction with the correlation-consistent valence polarized basis set aug-cc-pVTZ. In some cases, the structures were optimized with the hybrid meta exchange-correlation functional M06–2X in conjunction with 6–311+G(d,p). , Vibrational frequency analysis was used to determine the nature of stationary points: a minimum in the absence of imaginary frequencies or a saddle point if one frequency and only one frequency is imaginary. Furthermore, intrinsic reaction coordinate (IRC) calculations , have been performed for each saddle point geometry to confirm that it corresponds to a true transition state between two minima. After optimization, the energies of the stationary points were computed with coupled-cluster CCSD(T) − and aug-cc-pVTZ basis set to achieve more accurate energetics. All energies presented in this paper are the CCSD(T) energies corrected with zero-point energies from frequency calculations. The T1 diagnostic values for all species included in the minimum energy path, for both the C-side and N-side addition, support the adequacy of single-reference methods such as CCSD(T) (see Table S1).
Finally, for the two most important reaction pathways originating from the C-side and N-side addition of CN to the triple C–C bond of HC3N, more accurate single-point energy calculations were performed at the CCSD(T) level, corrected with a density fitted (DF)MP2 extrapolation to the complete basis set (CBS) and with corrections for core electron excitations. In particular, the energies were computed as
| 3 |
with
and where E(DF-MP2/CBS) is defined as
| 4 |
The E(DF-MP2/CBS) extrapolation was performed using Martin’s two-parameter scheme.
3. Results
3.1. Results of the Crossed-Molecular Beam Experiments
Reactive scattering signals were recorded at m/z = 76, corresponding to the parent ion of products with the gross formula C4N2. Since the parent peak is largely dominant in the electron ionization of dicyanoacetylene and there was no gain in the signal-to-noise ratio when using the soft ionization approach, , all the measurements were performed at m/z = 76 using an energy of 70 eV for the ionizing electrons. The product laboratory angular distribution detected at m/z = 76, together with the relevant velocity vector (“Newton”) diagram, is reported in Figure . The error bars (representing ± 1 standard deviation) are also shown when they exceed the size of the dots, indicating the intensity averaged over the different scans. In the Newton diagram of Figure , the Newton circle relative to NC4N scattered by the H coproduct is drawn on the assumption that all the available energy is converted into product translational energy for the reaction of CN in its ground rovibrational level (v = 0, N = 0). The Newton circles relative to the reactions involving the excited vibrational levels are very similar and are not shown for the sake of simplicity. The Newton circles delimit the LAB angular range within which NC4N can be scattered. Since in our previous experiments on the CN + C2H2 and CN + C2H4 reaction, we had experimental evidence of the formation of the isocyano products (with a small yield), we also considered the possibility that CNC3N could be formed in an H-displacement mechanism. The quite different enthalpy change of the two channels (see section ) implies a very different extension of the Newton circles, with the angular distribution of CNC3N confined between Θ = 27° and 33°. No apparent features can be associated with a contribution of this kind.
The TOF spectra measured at selected angles are reported in Figure .
2.

Time-of-flight (TOF) distributions of NC4N (m/z = 76) product detected at selected LAB scattering angles using a 70 eV electron energy. Counting times: 170 min at 22°, 120 min at 30°, and 180 min at 38°, respectively. The empty circles indicate the experimental data, while the solid black lines represent the distributions obtained with the best-fit functions shown in Figure .
The angular distribution extends for 30° and has a bell-shaped curve around the peak, which is located at the center-of-mass angle. The TOF distributions were recorded at ΘCM and in the forward (Θ = 22°) and backward (Θ = 38°) directions. Also, in the case of TOF spectra, no features are visible that could be associated with the channel leading to CNC3N + H. If it were present, the TOF distribution recorded at (Θ = 30°) would show a distinct component not present in the other two TOF spectra.
The solid lines in Figures and represent the curves calculated with the best-fit functions reported in Figure . The best-fit CM angular distribution (top panel of Figure is isotropic, with equal intensity from 0° to 180°. A slightly polarized T(θ) (with T(90°) = 0.7) or sideways T(θ) (with T(90°) = 1.3) still affords an acceptable fit of the experimental distributions (see the shaded areas in Figure ). The T(θ) shape is consistent with the formation of a bound intermediate with a lifetime longer than its rotational period.
3.
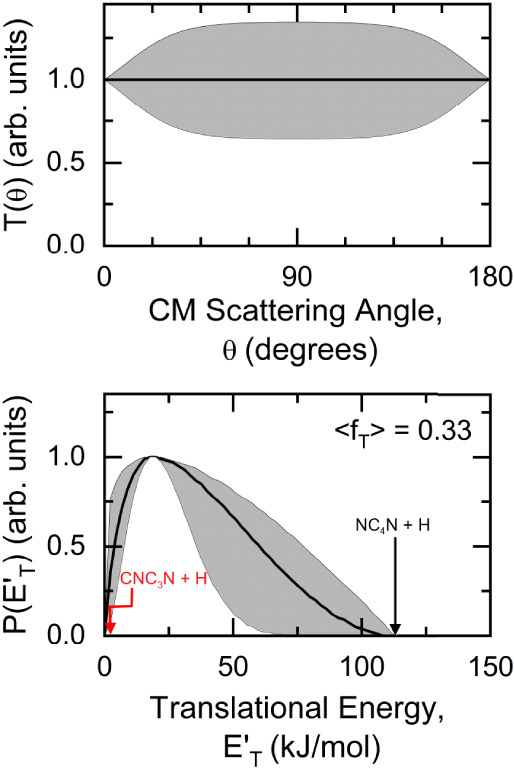
Best-fit CM product angular distribution (top) and translational energy distribution (bottom) for the NC4N + H channel. In the bottom panel, the black arrow indicates the total available energy for NC4N + H when considering the reaction of CN in its ground rovibrational level. The red arrow, instead, indicates the total available energy for the CNC3N + H isomeric channel. The shaded areas indicate the error bars for the best-fit CM functions.
Regarding the best-fit product translational distribution, P(E’T), shown in Figure (bottom panel), we observe that the peak is centered around 19 kJ/mol, while the tail extends to approximately 110 kJ/mol. The peak position suggests the presence of a small exit barrier. We recall that in this kind of experiment, the fit of both angular and TOF distributions is very sensitive to the rise and the peak position, while it is less sensitive to the tail of the P(E’T) (in this case, from E’T = 55 kJ/mol). Nonetheless, it is quite clear that the extra amount of energy carried by the CN vibrational excitation (which is quite sizable, being ΔV 1–0 = 24.4 kJ/mol, ΔV 2–0 = 48.6 kJ/mol, ΔV 3–0 = 72.4 kJ/mol) is not necessary to fit the experimental distributions (the indicated black arrow in Figure refers to the total available energy for the reaction channel leading to NC4N + H and involving CN in v = 0). The average product translational energy, defined as , is about 37 kJ/mol. We recall that the total available energy (ETot) is the sum of the collision energy, internal energy of the reactants (Eint), and the reaction exothermicity (− ). We considered here the value of that we calculated at the highest level of theory, that is, −64.4 kJ/mol. When considering ETot for the reaction involving CN(v = 0) and leading to the products NC4N + H, the average fraction of total available energy released into translation is 0.33, a typical value considering the energy profile of the PES as far as the pathway leading to NC4N + H is concerned.
As noted, the additional energy from the rovibrational excitation of CN does not need to be considered in the experimental distributions. This implies that the reaction is vibrationally adiabatic (the initial vibrational excitation of the CN reactant is retained as such in the final products and is not converted into translational energy of the products), as already observed in related reactions, including CN + C2H2, CN + CH3CCH, and CN + C2H4. ,
Concerning the possible occurrence of the channel leading to the isomer CNC3N, we note that, according to our theoretical calculations (see the next section), the is +42.3 kJ/mol at our best level of theory. Therefore, considering the E c of 44.8 kJ/mol, this channel is energetically permitted for only 2.5 kJ/mol. If a channel with this small amount of ETot were open, even with a very low yield, we would see a strong signal associated with it because of the large enhancement produced by the transformation Jacobian, which strongly amplifies the slow products. A centroid distribution around ΘCM would be clearly visible. In Figure S1, the simulated angular and TOF distributions when using a P(E’T) compatible with this channel (also reported in Figure S1) are shown as an example. The presence of excited rovibrational levels of CN is not expected to alter this since in the CN + C2H4 reaction, where we were able to disentangle the contribution of the isocyanoethylene channel, there was again no indication that the internal energy content of CN was converted into product translational energy. According to our PES calculations, an exit barrier of 64.9 kJ/mol is present above the energy of the reactants for this channel. Therefore, excited CN radicals in v = 2 and v = 3 do have enough energy to overcome the barrier. However, it is well known that vibrational excitation of the CN radical is not suitable for promoting this less favorable reaction channel (see, for instance, kinetic experiments on the related systems CN + C2H2, CN + C2H6, and CN + CH4). ,
3.2. Electronic Structure Calculations
The structures (interatomic distances, bond angles, and dihedral angles) of the reactants, possible products, and stationary points of the PES are shown in Figures –. Most geometric configurations have been optimized at the B3LYP/aug-cc-pVTZ level, with the exception of the van der Waals complex (vdW) in the entrance channel and the transition state (TS) that connects vdW with the addition intermediate INT1, which have been computed at M062X/6–311+G (d,p). Figures and depict the two portions of the PES that originate when the CN radical interacts with cyanoacetylene on the C-side and the N-side, respectively. All reported energy values are at the CCSD (T)/aug-cc-pVTZ level and include ZPE. We have characterized the lowest energy paths (in red in Figures and ) for the two portions of the PES, also at the higher CBS level, to provide a better evaluation of the energy values as they are critical to interpreting the outcome of the CMB experiments.
4.
Geometries of reactants and products were optimized at the B3LYP/aug-cc-pVTZ level. Bond lengths (in Å) are displayed in black, and bond angles (in degrees) are in blue. Carbon atoms are represented in gray, hydrogen atoms in white, and nitrogen atoms in blue.
6.
Geometries of saddle points optimized at the B3LYP/aug-cc-pVTZ level. TS vdW-INT1 was optimized at the M062X/6–311+G(d,p) level. The units of distance and angle and the color scheme are the same as in Figure .
7.
Portion of the potential energy surface with products resulting from the addition of the C-side of the cyano radical. Energies (kJ/mol) in black were computed at the CCSD(T)//B3LYP/aug-cc-pVTZ level. Energies (in kJ/mol) are in green at the CCSD(T)//M062X/6–311+G(d,p) level. Shown in red is the most favorable pathway, with the energy values calculated also at the CBS level.
8.
Potential energy surface with products resulting from the addition of the N-side of the cyano radical. Energies (kJ/mol) were computed at the CCSD(T)//B3LYP/aug-cc-pVTZ level. Shown in red is the most favorable pathway, with the energy values calculated also at the CBS level.
5.
Geometries of intermediates optimized at the B3LYP/aug-cc-pVTZ level. The vdW geometry was optimized at the M062X/6–311+G (d,p) level. The units of distance and angle and the color scheme are the same as in Figure .
The interaction of the cyano radical with cyanoacetylene, from both the C-side and N-side, can occur via: (a) the attack on the π-system of the C–C triple bond; (b) the attack on the π-system of the C–N triple bond; and (c) H-abstraction. In the case of the interaction from the C-side, addition to the lone pair of N of the HC3N cyano group is also possible.
A concise description of the reaction mechanism for all of the reaction channels is provided below.
Addition of the cyano radical on the C-side can occur in four different ways: (1) The interaction with the C–C triple bond can lead to the CN addition on the unsubstituted acetylenic carbon, resulting in the formation of INT1-t that easily isomerizes to the cis isomer INT1-c; both isomers can undergo H-loss by overcoming a barrier (TS INT1t-P1A and TS INT1c-P1A, respectively), located at ca. 21 kJ/mol above the energy of the NC4N + H products. This is the only exothermic reaction channel of the entire PES. The entrance channel is characterized by the formation of a van der Waals adduct (vdW) that must overcome a small barrier (TS vdW-INT1) to rearrange into INT1-t. This portion of the PES is crucial for determining the reaction rate coefficient. The energy values obtained in our calculations are in line with those of previous theoretical evaluations, falling within the uncertainty of the employed methods. Since the entrance channel has already been the subject of dedicated calculations, , we did not devote additional effort to this part of the PES, considering that our CMB experiments were conducted at an E c value that is quite high. All the factors that control the k(T) values have already been discussed at length in refs. , . The dissociation of INT1-t into CN + CCHCN (P1B) was also considered; however, the enthalpy change is so high ( relative to the reactants) that we can consider it as not accessible under most conditions of interest. The addition on the C–C triple bond can also lead to INT2 if the entering CN group attaches to the carbon already bound to a cyano group in the HC3N coreactant. INT2 can only dissociate into H + CC(CN)2 (P2) in a very endothermic channel which is, therefore, not open under most conditions of interest. (2) The CN radical can also add to the C atom of the HC3N cyano group, leading to the INT3 intermediate. In this case, a significant entrance barrier (TS R-INT3) of 21.2 kJ/mol is present. The breaking of the H–C bond of INT3 leads to the formation of a cyclic CC(N)CCN isomer in a very endothermic channel , while the C–C σ bond fission leads to NCCN + HCC (P3B), another endothermic channel with . An exit barrier associated with TS INT3-P3B (located 74.2 kJ/mol above the energy of the reactants) must be overcome. (3) The last addition mechanism is the one in which the CN radical adds to the N atom of HC3N, leading, in a barrierless process, to the formation of INT4. However, INT4 can only dissociate to CCCNCN + H (P4A) and CNCN + HCC (P4B) products in two endothermic channels ( and .
The H-abstraction channel leading to HCN + C3N (P9) is endothermic by 48.2 kJ/mol.
Unlike many other CN reactions with unsaturated hydrocarbons, ,, the attacks of CN on the N-side are characterized by an entrance barrier for all possible addition sites. More specifically, (4) the addition to the triple C–C bond occurs with an entrance barrier of 27.9 kJ/mol and leads to INT5 in its cis and trans form (more stable than the reactants by ca. 137 kJ/mol); INT5 can dissociate into CNCCCN + H (P5A) through TS INT5-P5A (located at +64.9 kJ/mol at the CBS level of calculations), an endothermic channel with . INT5 can also undergo isomerization to INT8 (by overcoming a barrier located at +73.2 kJ/mol with respect to the energy asymptote of the reactants) that, in turn, can dissociate into CNCCCN + H (P5A) or CN + HCCNC (P6B) in a very endothermic channel . Alternatively, the addition of the C–C triple bond can also lead to INT6, in which the new CN group is attached to the carbon already bound to a cyano group. In this case, the entrance barrier is even higher (+ 50.4, TS R-INT6), while INT6 can dissociate into CC(CN)(NC) + H (P6A) products or the already cited CN + HCCNC (P6B) products. (5) Furthermore, the CN addition on the N-side can involve the C atom of the CN group of cyanoacetylene, but the entrance barrier is very high (+ 69.4 kJ/mol, TS R-INT7), and the addition intermediate INT7 can only dissociate into very high-energy fragments, namely the channel CNCN + HCC (P4B) and cyclic-CC(N)CNC + H (P7) .
In the case of CN approaching from the N-side, the H-abstraction leads to products (C3N + HNC) in a very endothermic channel (+107.9 kJ/mol) and through a barrier of 144.7 kJ/mol.
The present results are in line with the previous calculations by Petrie and Osamura who used the CCSD(T)/aug-cc-pVXZ//B3-LYP/6–311G** (X) D,T) levels of theory to derive the PES for the C3N + HNC reaction, which has some common points with the PES investigated here. For instance, from their PES, an enthalpy change of −58.6 kJ/mol can be inferred for the CN + HC3N → NC4N + H reaction, in good agreement with our value of −64.4 kJ/mol at the CBS level. The energy content of INT1-t (int63 in their scheme of Figure is −231.1 kJ/mol, which can be compared with our CBS value of −232.8 kJ/mol. The equivalent of our TS INT1t-P1A falls at −39.5 kJ/mol. Their geometries are also very similar.
To summarize, the evaluation of the energetic profile of the global PES reveals that only one exothermic channel is open, namely, the one that leads to dicyanoacetylene via an H-displacement mechanism. All of the other channels are endothermic, and some of them are also characterized by high energy barriers.
4. Discussion
The CMB data clearly suggest that an H-displacement channel leading to the formation of molecular products with a C4N2 gross formula is open. The experimental determination of the product energy release is fully consistent with the formation of dicyanoacetylene. The absence of any structure in both the LAB distributions and the best-fit CM functions indicates that no high-energy additional channels are open. Considering the energetics of the other possible H-displacement channels, as they result from the present electronic structure calculations, only the channel leading to 3-isocyano-2-propynenitrile (P5A) is barely accessible (by ca. 2 kJ/mol) under our experimental conditions (without considering the entrance barrier). Were this channel were to occur, features in the LAB distributions would be clearly visible due to the strong enhancement provided by the CM→LAB Jacobian to products with low CM speeds (see Figure S1). In similar cases, ,− contributions accounting for 5% of the total yield were clearly identified and separated during the data analysis. On the contrary, here a single-contribution fit with a backward-forward symmetric T(θ) and a smooth bell-shaped P(E’T) nicely reproduces all the experimental observables.
The experimental results are perfectly in line with the main characteristics of the PES minimum energy path. The formation of stable intermediates (cis/trans INT1) with a deep potential aligns well with the backward-forward symmetric CM angular distribution, which implies the formation of a long-lived complex. The barrier on the exit channels from cis/trans INT1 has a height of 21 kJ/mol, perfectly consistent with the position of the peak of the best-fit P(E’T) at 19 kJ/mol.
Concerning the possible role of the internal excitation of CN on the scattering properties, as in previous studies from our laboratory, ,, as well as kinetics experiments , on similar systems, the vibrational excitation of CN was not seen to promote the reaction and was not converted into product translational energy but was retained as vibrational excitation of the molecular products.
The rotational excitation of CN could affect the reaction as it was previously observed for the related CN + C2H4 reaction. In that case, it was speculated that CN rotational excitation enhances the yield of the CH2CHNC channel over that of the favored CH2CHCN one. However, unlike that case, the formation of 3-isocyano-2-propynenitrile (P5A) is strongly unfavored due to the large endothermicity and high barrier in the exit channel (TS INT5-P5A).
The results obtained in this study can be compared with the data reported in the literature for similar systems and, in particular, with the reaction CN + C2H2 since cyanoacetylene can be regarded as a functionalized acetylene with a nitrile group in place of one of the two hydrogen atoms. , Such a comparison is particularly appropriate when carried out with the work by Casavecchia et al. and Leonori et al., given the fact that the CN + C2H2 reaction was investigated using the same CMB apparatus, CN beam, and at a very similar collision energy of E c = 48.1 kJ/mol. For the PES comparison, we can refer to the work by Huang et al. Starting from the experiments, the CM angular distributions are clearly different: in the case of CN + C2H2, the best-fit T(θ) shows some forward bias, indicating the shortening of the intermediate lifetime, while the T(θ) of the present determination is fully backward-forward symmetric. This is well explained by considering the reduced exothermicity of the channel leading to NC4N + H with respect to the analogous channel leading to HC3N and the much higher exit barrier (21 kJ/mol with respect to the products, compared with +7 kJ/mol for CN + C2H2 for the most favorable channel). In addition, the presence of an additional CN group in the molecular skeleton of the reactant results in an increased number of degrees of freedom among which to distribute the large amount of energy liberated by the formation of the bound intermediates (ca. 230–240 kJ/mol in both cases).
Also, the shape of the best-fit P(E’T) is quite different: in the case of the CN + C2H2, the peak is flat from E’T = 20–60 kJ/mol. This characteristic suggests that two mechanisms actually contribute to the reactive signal. The second contribution was, indeed, associated with the formation of isocyanoacetylene + H, a channel that is accessible under the conditions of the experiment by Leonori et al.
The similarities and differences in the PES of CN + C2H2 vs CN + HC3N do not only affect the reaction dynamics, but have a direct impact on the reaction kinetics as well. As already noted by Halpern et al., at room temperature, the CN + HC3N reaction is significantly slower than the CN + C2H2 reaction. We now know that this is not caused by the presence of an entrance barrier, but rather associated with the effect of a vdW complex in the entrance channel and the submerged transition state vdW-INT1. , Furthermore, we note that the addition of CN to the triple C–C bond on the N-side is barrierless in the case of the CN + C2H2 reaction. The HCCHNC intermediate easily isomerizes to the HCCHCN intermediate, which then dissociates into HC3N + H. In the title reaction, instead, the N-side addition reactive flux is lost in a repulsive channel.
When comparing the present results with the related CN + C2H3CN reaction, which was also investigated in our laboratory using the same experimental and theoretical approaches, some similarities can be noted. Also in this case, there was no evidence that the vibrational excitation of the CN radicals is converted into product translational energy, and the isocyano products are not formed (their formation channels are endothermic and characterized by high exit barriers). However, a small yield of 1,1-dicyanoethylene was observed in an exothermic channel characterized by submerged transition states. This is in contrast to the current system, where the equivalent path (INT2 → TS INT2-P2 → CC(CN)2 + H) is strongly endothermic.
A final comparison should be made with the CN + HCN reaction since cyanopolyynes are often compared to hydrogen cyanide. The CN + HCN reaction has been studied using experimental and theoretical methods. ,,, Experimental studies of chemical kinetics consistently indicate the presence of a significant activation energy. , The presence of a barrier in the entrance channel was confirmed by theoretical calculations, ,, even when considering the favored attack, i.e., the addition of the CN radical to the carbon atom of HCN. Therefore, the situation is very different from that of the reaction under consideration. The reason for this is the greater strength of the C–N triple bond compared to the C–C triple bond, which is due to the electronegativity of nitrogen and its smaller size, enhancing orbital overlap. These effects can be easily appreciated by observing the structure of cyanoacetylene (see Figure ), in which the CC bond distance is 1.201 Å (1.2 Å is the typical value for acetylenic compounds), while the CN distance is 1.155 Å (1.15–1.16 Å are the typical values for nitriles). This is reflected in their differing reactivity in bimolecular reactions: while it is known that CN, or other radicals, readily add to triple C–C bonds (e.g., CN + C2H2, CH3CCH), ,, the cyano group present in nitriles is rarely involved in reactions that cause its breaking and is preserved in the products, even in the case of reactions with very reactive species like atomic nitrogen in the 2D electronically excited state (e.g., N(2D) + CH3CN, N(2D) + C2H3CN, and N(2D) + HC3N). ,,, The characteristics of the cyano group in nitriles are so unique that cyano is considered a pseudohalogen.
5. Implications for Astrochemistry and Cosmochemistry
Our work presents experimental evidence that NC4N is the sole molecular product formed in the only open H-displacement channel. 3-Isocyano-2-propynenitrile is not formed with a measurable yield within the sensitivity of our technique, even though the total energy available to the reactants, when considering the collision energy and the internal energy of the CN radical, is sufficient to overcome the exit barrier and to compensate for the positive enthalpy change associated with that channel. The other possible reaction channels are even more endothermic and are not seen to occur under the conditions in our experiments. It is not straightforward to convert the collision energy of CMB experiments into temperature. Based on previous work, it can be inferred that the collision energy of this experiment corresponds to a temperature between 300 and 1000 K. , Obviously, at the low temperatures of relevance to the atmosphere of Titan or cold interstellar regions, NC4N + H is the only possible set of products.
After the determination of the rate coefficient at low temperature, the title reaction has been considered in the photochemical models of the atmosphere of Titan as a possible formation route of dicyanoacetylene, together with other, less-characterized reactions (e.g., N + HC4N or C3N + HCN). , However, photochemical models still underpredict the amount of NC4N and the related species C2N2, , pointing to the fact that the chemistry of these two species has not yet been well understood.
On the contrary, the CN + HC3N reaction is not mentioned as a possible formation route of NC4N in TMC-1 by Agúndez et al. Agúndez et al. were only referring to the reactions proposed by Petrie and Osamura and Petrie et al. for C2N2 and NC4N.
| 5 |
| 6 |
However, considering the large fractional abundance of both CN and HC3N in TMC-1 (both in the order of 10–8) and the large rate coefficient measured at low T, the title reaction is clearly an important formation route of dicyanoacetylene that should be considered in astrochemical models. In the recent upgraded version of the UMIST Database for Astrochemistry, the CN + HC3N reaction has been included in both the TMC-1 and IRC+10216 models using the fit expression of the CRESU data and assuming that NC4N + H is the sole reaction channel. The resulting simulated fractional abundance of NC4N+ calculated at 1.6 × 105 years for the O-rich model or at 1.0 × 106 years for the C-rich model is in good agreement with the value derived by Agúndez et al.
Interestingly, the title reaction is considered a termination step in the sequence of reactions that lead to the formation of long cyanopolyynes (the largest observed one is HC11N) in the interstellar medium and circumstellar envelopes (see Cheikh Sid Ely et al. and references therein):
| 7 |
| 8 |
| 9 |
| 10 |
To verify whether our results for the title reaction can be extended to other reactions in the CN + H–(CC−) n CN series, we performed calculations for the CN + HC5N reaction for the minimum energy pathway only at the CCSD (T)//B3LYP/aug-cc-pVTZ and CBS levels of theory (determining the full PES, as presented in Figures and F require dedicated work and a separate publication). Again, we did not find an entrance barrier above the energy of the reactants; the reactants correlate with a covalent intermediate (INT9 inFigure ), and its dissociation into the NC6N + H products proceeds with a barrier that remains well below the energy of the reactants. Therefore, this reaction will be characterized by a rate coefficient in the gas kinetics limit (in the 10–10 cm3 s–1 range) even at the very low temperatures of interest in the ISM or the atmosphere of Titan.
9.
Minimum energy path in the potential energy surface for the CN + HC5N reaction. Energies are computed in kJ/mol at the CCSD(T)//B3LYP/aug-cc-pVTZ (black) and CBS (red) levels.
The formation of NC(CC) n CN (including dicyanoacetylene with n = 1) stops further growth in the eqs – chain since dicyanopolyynes are unlikely to be reactive toward further attack by CN or C2H, as suggested by Cheikh Sid Ely et al., who mentioned the low rate coefficient for the reaction CN + NC4N at room temperature (5.4 × 10–13 cm3 s–1) and their preliminary calculations (unpublished to the best of our knowledge) on the entrance barrier of CN + NC4N and C2H + NC4N. Since this aspect is crucial to understanding the growth and destruction routes of cyanopolyynes, we have also carried out calculations for the entrance channel of the last reaction. As visible in Figure , all of the possible addition sites of C2H to NC4N are indeed characterized by a significant entrance barrier that would make this reaction impossible in the low T regions of the interstellar medium. However, this does not necessarily mean that an environment with abundant CN radicals will impede the growth of cyanopolyynes by converting them into dicyanopolyynes: a recent astrochemical model revealed that HC5N is formed essentially by the reactions N + C6H, C2H + HC3N, C3N + C2H2, and C4H + HCN in cold clouds and C3N + C2H2 in shocked regions. In the same work, the CRESU rate coefficients for the reactions C2H + HC3N and C3N + C2H2 were provided (for temperatures as low as 24 K), as well as the potential energy surface with a kinetic analysis for three other possible formation routes of HC5N (N + C6H, C4H + HCN, and C4H + HNC). Therefore, the importance of reaction (8) in the formation of larger cyanopolyynes seems to be reduced, at least in the case of HC5N, but larger cyanopolyynes could be formed in similar ways.
10.
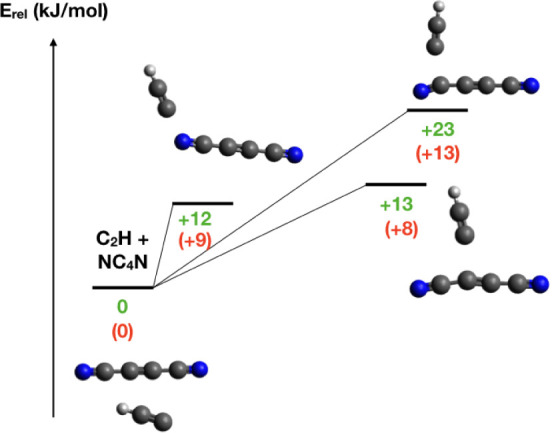
Entrance channels of the potential energy surface for C2H + NC4N. Energies (in kJ/mol) are in green at the CCSD(T)/aug-cc-pVTZ//M062X/6-311+G(d,p) level and in red at the CBS level.
From the global PES that we have derived in this work, we can also infer some properties of other reactions that have not been investigated previously (to the best of our knowledge). For instance, the reaction C2H + C2N2 is characterized by a significant entrance barrier (+ 19.7 kJ/mol), and, therefore, it cannot occur either in the interstellar medium or in the atmosphere of Titan. On the contrary, the C2H + CNCN reaction is barrierless and easily produces HC3N + CN. It can therefore be considered either a destruction route of isocyanogen or an alternative formation route of HC3N (provided that isocyanogen is available).
Finally, it is worth noticing that the reaction CN + isocyanoacetylene (P6B in the scheme of Figure ) is barrierless in the entrance channel and can easily form both 3-isocyano-2-propynenitrile + H or cyanoacetylene + CN according to the sequences:
CN + HCCNC (isocyanoacetylene) → INT8 (−246 kJ/mol) → TS INT8-P5A (−43.2 kJ/mol) → CNC3N (3-isocyano-2-propynenitrile) + H (−72.1 kJ/mol)
CN + HCCNC (isocyanoacetylene) → INT6 (−211.1 kJ/mol) → TS R-INT6 (−59.3 kJ/mol) → CN + HC3N (−109.7 kJ/mol)
where the energy values are those calculated at the CCSD(T)//B3LYP/aug-cc-pVTZ level of theory and assuming the energy content of CN + HCCNC as the zero of the energy scale. Since TS R-INT6 is lower in energy than TS INT8-P5A, the formation of cyanoacetylene + CN is favored. In other words, the reaction of CN with isocyanoacetylene is an efficient way to convert it to cyanoacetylene with a null consumption of CN radicals. This can be generalized for all reactions of the type CN + isocyanopolyynes, a new class of reactions that could help better understand the relationship between the abundance of cyanopolyynes and isocyanopolyynes. For instance, in TMC-1 [HC3N]/[HCCNC] is 77.0 ± 8.0, while in IRC+10216, it is 392 ± 22. Considering the next member of the series, [HC5N]/[HC4NC] is 600 ± 70 in TMC-1 and ≥ 2000 in IRC+10216. The origin of the large difference between the TMC-1 and IRC+10216 values is not yet known nor is the variation along the series.
We recommend testing the conversion of isocyanopolyynes into their cyano counterparts through reactions with CN in astrochemical models. In CN-rich environments, we expect that the cyanopolyynes are converted into dicyanopolyynes, but at the same time, isocyanopolyynes are mostly converted into cyanopolyynes. The role of these two contrasting trends can only be assessed in astrochemical models. A recent model proposed by Xue et al. for TMC-1 overproduces both HCCNC and HC4NC. In that model, an important destruction route for both cyanopolyynes and isocyanopolyynes is the reaction with atomic carbon, which is considered to occur with the same efficiency in both cases (see also Loison et al. and Li et al.). Since the CN radicals can be as abundant as C atoms, the reactions of cyanopolyynes and isocyanopolyynes are expected to be important destruction routes but with a very different outcome. For instance, the increased fractional abundance of CN radicals in the CSEs of AGB stars (such as IRC+10216) of at least 1 order of magnitude wrt TMC-1 could explain why isocyanopolyynes are almost absent, even though their formation routes are the same as those considered for TMC-1.
6. Conclusions
In this work, we present an experimental and theoretical study of the reaction between the cyano radical and cyanoacetylene. Based on experimental evidence and electronic structure calculations of the relevant potential energy surface, the reaction involving cyanoacetylene and the cyano radical leads to only one exothermic channel associated with the formation of dicyanoacetylene. Furthermore, we derive some properties of the related reactions C2H + CNCN (isocyanogen) and CN + HCCNC (isocyanoacetylene): the C2H + CNCN reaction leads to the formation of CN + HC3N, and the main channel of the CN + HCCNC reaction also leads to CN + HC3N. This last reaction efficiently converts isocyanoacetylene and, by extension, any isocyanopolyyne into their cyano counterparts without a net loss of cyano radicals. The effects of this new family of reactions should be tested in astrochemical models.
Finally, we also characterized the CN + HC5N reaction (confirming that it easily evolves toward NC6N + H) and the entrance channel of the reaction C2H + NC4N. The addition of C2H to all possible sites of NC4N is characterized by a significant entrance barrier, thus confirming that, once formed, dicyanoacetylene terminates the growth of cyanopolyynes.
Supplementary Material
Acknowledgments
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 811312 for the project “Astro-Chemical Origins” (ACO). Support from the Italian Space Agency (Bando ASI Prot. n. DC-DSR-UVS-2022-231, Grant No. 2023-10-U.0 MIGLIORA) is also acknowledged. MR and NFL acknowledge financial support under the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.1, Call for Tender No. 104 published on 2.2.2022 by the Italian Ministry of University and Research (MUR), funded by the European Union – NextGenerationEU – Project Title 2022JC2Y93 ChemicalOrigins: linking the fossil composition of the Solar System with the chemistry of protoplanetary disks – CUP J53D23001600006 – Grant Assignment Decree No. 962 adopted on 30.06.2023 by the Italian Ministry of University and Research (MUR). EVFA and NFL thank the Herla Project (http:// hscw.herla.unipg.it) – Università degli Studi di Perugia for allocated computing time. The authors thank the Dipartimento di Ingegneria Civile e Ambientale of the University of Perugia for allocating computing time within the project “Dipartimenti di Eccellenza 2018–2022, F. Ferlin for the synthesis of cyanoacetylene, and P. Recio and D. Marchione for assistance in running CMB experiments. NB acknowledges additional support by the International Space Science Institute (ISSI) in Bern, through ISSI International Team project #615 “Advancing Titan’s Atmospheric Chemistry Knowledge”.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsearthspacechem.5c00154.
Simulation of the laboratory distributions using CM functions for the CNC3N + H channel T1 analysis (PDF)
#.
CEA, DES, ISEC, DMRC, Univ. Montpellier, Marcoule, France
The authors declare no competing financial interest.
Published as part of ACS Earth and Space Chemistry special issue “Eric Herbst Festschrift”.
Footnotes
NIST Mass Spectrometry Data Center Collection (C) 2014, copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
For clarity, only the cis isomer is shown in Figure .
In the reaction, a second intermediate can be formed with the structure CH2CCN via a 1,2 H-shift; this channel gives a sizable contribution to the reaction. An analogous process for the title reaction would require a much more complex CN-shift and does not occur.
References
- Lee K. L. K., Loomis R. A., Burkhardt A. M., Cooke I. R., Xue C., Siebert M. A., Shingledecker C. N., Remijan A., Charnley S. B., McCarthy M. C.. et al. Discovery of Interstellar trans-cyanovinylacetylene (HC ? CCH = CHC ? N) and vinylcyanoacetylene (H2C = CHC3N) in GOTHAM Observations of TMC-1. Astrophys. J. Lett. 2021;908:L11. doi: 10.3847/1538-4357/abd08b. [DOI] [Google Scholar]
- Shingledecker C. N., Lee K. L. K., Wandishin J. T., Balucani N., Burkhardt A. M., Charnley S. B., Loomis R., Schreffler M., Siebert M., McCarthy M. C., McGuire B. A.. Detection of interstellar H2CCCHC3N. A possible link between chains and rings in cold cores. Astron. Astrophys. 2021;652:L12. doi: 10.1051/0004-6361/202140698. [DOI] [Google Scholar]
- McGuire B. A., Loomis R. A., Burkhardt A. M., Lee K. L. K., Shingledecker C. N., Charnley S. B., Cooke I. R., Cordiner M. A., Herbst E., Kalenskii S., Siebert M. A., Willis E. R., Xue C., Remijan A. J., McCarthy M. C.. Detection of two interstellar polycyclic aromatic hydrocarbons via spectral matched filtering. Science. 2021;371:1265–1269. doi: 10.1126/science.abb7535. [DOI] [PubMed] [Google Scholar]
- Cernicharo J., Cabezas C., Fuentetaja R., Agúndez M., Tercero B., Janeiro J., Juanes M., Kaiser R. I., Endo Y., Steber A. L., Pérez D., Pérez C., Lesarri A., Marcelino N., de Vicente P.. Discovery of two cyano derivatives of acenaphthylene (C12H8) in TMC-1 with the QUIJOTE line survey. Astron. Astrophys. 2024;690:L13. doi: 10.1051/0004-6361/202452196. [DOI] [Google Scholar]
- Wenzel G.. et al. Detection of interstellar 1-cyanopyrene: A four-ring polycyclic aromatic hydrocarbon. Science. 2024;386:810–813. doi: 10.1126/science.adq6391. [DOI] [PubMed] [Google Scholar]
- Wenzel G.. et al. Discovery of the Seven-ring Polycyclic Aromatic Hydrocarbon Cyanocoronene (C24H11CN) in GOTHAM Observations of TMC-1. Astrophys. J. Lett. 2025;984:L36. doi: 10.3847/2041-8213/adc911. [DOI] [Google Scholar]
- Cordiner M. A., Nixon C. A., Charnley S. B., Teanby N. A., Molter E. M., Kisiel Z., Vuitton V.. Interferometric Imaging of Titan’s HC3N, H13CCCN, and HCCC15N. Astrophys. J. Lett. 2018;859:L15. doi: 10.3847/2041-8213/aac38d. [DOI] [Google Scholar]
- Kunde V., Aikin A., Hanel R., Jennings D., Maguire W., Samuelson R.. C4H2, HC3N and CH2NH2 in Titan’s atmosphere. Nature. 1981;292:686–688. doi: 10.1038/292686a0. [DOI] [Google Scholar]
- Perera-Jarmer, M. A. ; Khanna, R. K. ; Samuelson, R. E. . C4N2 on Titan. In Bulletin of the American Astronomical Society; American Astronomical Society, 1986; pp. 808. [Google Scholar]
- Calcutt H., Jørgensen J. K., Müller H. S. P., Kristensen L. E., Coutens A., Bourke T. L., Garrod R. T., Persson M. V., van der Wiel M. H. D., van Dishoeck E. F., Wampfler S. F.. The ALMA-PILS survey: complex nitriles towards IRAS 16293–2422. Astron. Astrophys. 2018;616:A90. doi: 10.1051/0004-6361/201732289. [DOI] [Google Scholar]
- Jaber al-Edhari A., Ceccarelli C., Kahane C., Viti S., Balucani N., Caux E., Faure A., Lefloch B., Lique F., Mendoza E., Quenard D., Wiesenfeld L.. History of the solar-type protostar IRAS 16293–2422 as told by the cyanopolyynes. Astron. Astrophys. 2017;597:A40. doi: 10.1051/0004-6361/201629506. [DOI] [Google Scholar]
- Vastel C., Loison J. C., Wakelam V., Lefloch B.. Isocyanogen formation in the cold interstellar medium. Astron. Astrophys. 2019;625:A91. doi: 10.1051/0004-6361/201935010. [DOI] [Google Scholar]
- Bianchi E., Ceccarelli C., Codella C., López-Sepulcre A., Yamamoto S., Balucani N., Caselli P., Podio L., Neri R., Bachiller R., Favre C., Fontani F., Lefloch B., Sakai N., Segura-Cox D.. SOLIS. XV. CH3CN deuteration in the SVS13-A Class I hot corino. Astron. Astrophys. 2022;662:A103. doi: 10.1051/0004-6361/202141893. [DOI] [Google Scholar]
- Fontani F.. et al. Seeds of Life in Space (SOLIS). I. Carbon-chain growth in the Solar-type protocluster OMC2-FIR4. Astron. Astrophys. 2017;605:A57. doi: 10.1051/0004-6361/201730527. [DOI] [Google Scholar]
- Scibelli S., Shirley Y., Megías A., Jiménez-Serra I.. Survey of complex organic molecules in starless and pre-stellar cores in the Perseus molecular cloud. Mon. Not. R. Astron. Soc. 2024;533:4104–4149. doi: 10.1093/mnras/stae2017. [DOI] [Google Scholar]
- Belloche A., Garrod R. T., Müller H. S. P., Menten K. M.. Detection of a branched alkyl molecule in the interstellar medium: iso-propyl cyanide. Science. 2014;345:1584–1587. doi: 10.1126/science.1256678. [DOI] [PubMed] [Google Scholar]
- Rivilla V. M., Jiménez-Serra I., Martín-Pintado J., Colzi L., Tercero B., de Vicente P., Zeng S., Martín S., García de la Concepción J., Bizzocchi L., Melosso M., Rico-Villas F., Requena-Torres M. A.. Molecular Precursors of the RNA-World in Space: New Nitriles in the G + 0.693 - 0.027 Molecular Cloud. Front. Astron. Space Sci. 2022;9:876870. doi: 10.3389/fspas.2022.876870. [DOI] [Google Scholar]
- Nazari P., van Gelder M. L., van Dishoeck E. F., Tabone B., van’t Hoff M. L. R., Ligterink N. F. W., Beuther H., Boogert A. C. A., Caratti o Garatti A., Klaassen P. D., Linnartz H., Taquet V., Tychoniec Ł.. Complex organic molecules in low-mass protostars on Solar System scales. II. Nitrogen-bearing species. Astron. Astrophys. 2021;650:A150. doi: 10.1051/0004-6361/202039996. [DOI] [Google Scholar]
- Chapillon E., Dutrey A., Guilloteau S., Piétu V., Wakelam V., Hersant F., Gueth F., Henning T., Launhardt R., Schreyer K., Semenov D.. Chemistry In Disks. Vii. First Detection Of HC3N In Protoplanetary Disks. Astrophy. J. 2012;756:58. doi: 10.1088/0004-637X/756/1/58. [DOI] [Google Scholar]
- Ilee J. D.. et al. Molecules with ALMA at Planet-forming Scales (MAPS). IX. Distribution and Properties of the Large Organic Molecules HC3N, CH3CN, and c-C3H2 . Astrophys. J. 2021;257:9. doi: 10.3847/1538-4365/ac1441. [DOI] [Google Scholar]
- Bergner J. B., Guzmán V. G., Öberg K. I., Loomis R. A., Pegues J.. A Survey of CH3CN and HC3N in Protoplanetary Disks. Astrophy. J. 2018;857:69. doi: 10.3847/1538-4357/aab664. [DOI] [Google Scholar]
- Bachiller R., Fuente A., Bujarrabal V., Colomer F., Loup C., Omont A., de Jong T.. A survey of CN in circumstellar envelopes. Astron. Astrophys. 1997;319:235–243. [Google Scholar]
- Agúndez M., Cernicharo J., Quintana-Lacaci G., Castro-Carrizo A., Velilla Prieto L., Marcelino N., Guélin M., Joblin C., Martín-Gago J. A., Gottlieb C. A., Patel N. A., McCarthy M. C.. Growth of carbon chains in IRC+10216 mapped with ALMA. Astron. Astrophys. 2017;601:A4. doi: 10.1051/0004-6361/201630274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J., Li X., Sun J., Millar T. J., Zhang Y., Qiu J., Quan D., Esimbek J., Zhou J., Gao Y.. et al. A λ 3 mm Line Survey toward the Circumstellar Envelope of the Carbon-rich AGB Star IRC+ 10216 (CW Leo) Astrophys. J., Suppl. Ser. 2024;271:45. doi: 10.3847/1538-4365/ad2460. [DOI] [Google Scholar]
- Gong Y., Henkel C., Spezzano S., Thorwirth S., Menten K., Wyrowski F., Mao R., Klein B.. A 1.3 cm line survey toward IRC+ 10216. Astron. Astrophys. 2015;574:A56. doi: 10.1051/0004-6361/201424819. [DOI] [Google Scholar]
- Gensheimer P. D.. Observations of HCCNC and HNCCC in IRC+10216. Astrophys. Space Sci. 1997;251:199–202. doi: 10.1023/A:1000744924767. [DOI] [Google Scholar]
- Gensheimer P. D.. Detection of HCCNC from IRC+10216. Astrophys. J. Lett. 1997;479:L75–L78. doi: 10.1086/310576. [DOI] [Google Scholar]
- Siebert M. A., Van de Sande M., Millar T. J., Remijan A. J.. Investigating Anomalous Photochemistry in the Inner Wind of IRC+10216 through Interferometric Observations of HC3N. Astrophy. J. 2022;941:90. doi: 10.3847/1538-4357/ac9e52. [DOI] [Google Scholar]
- Cuadrado S., Goicoechea J. R., Cernicharo J., Fuente A., Pety J., Tercero B.. Complex organic molecules in strongly UV-irradiated gas. Astron. Astrophys. 2017;603:A124. doi: 10.1051/0004-6361/201730459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratier P., Pety J., Guzmán V., Gerin M., Goicoechea J. R., Roueff E., Faure A.. The IRAM-30 m line survey of the Horsehead PDR. III. High abundance of complex (iso-)nitrile molecules in UV-illuminated gas. Astron. Astrophys. 2013;557:A101. doi: 10.1051/0004-6361/201321031. [DOI] [Google Scholar]
- Ulich B. L., Conklin E. K.. Detection of methyl cyanide in Comet Kohoutek. Nature. 1974;248:121–122. doi: 10.1038/248121a0. [DOI] [Google Scholar]
- Bockelée-Morvan D., Lis D.C., Wink J.E., Despois D., Crovisier J., Bachiller R., Benford D.J., Biver N., Colom P., Davies J.K.. et al. New molecules found in comet C/1995 O1 (Hale-Bopp). Investigating the link between cometary and interstellar material. Astron. Astrophys. 2000;353:1101–1114. [Google Scholar]
- Fray N., Bénilan Y., Cottin H., Gazeau M.-C., Crovisier J.. The origin of the CN radical in comets: A review from observations and models. Planet. Space Sci. 2005;53:1243–1262. doi: 10.1016/j.pss.2005.06.005. [DOI] [Google Scholar]
- Hänni N., Altwegg K., Balsiger H., Combi M., Fuselier S. A., De Keyser J., Pestoni B., Rubin M., Wampfler S. F.. Cyanogen, cyanoacetylene, and acetonitrile in comet 67P and their relation to the cyano radical. Astron. Astrophys. 2021;647:A22. doi: 10.1051/0004-6361/202039580. [DOI] [Google Scholar]
- Rimmer P. B., Majumdar L., Priyadarshi A., Wright S., Yurchenko S. N.. Detectable Abundance of Cyanoacetylene (HC3N) Predicted on Reduced Nitrogen-rich Super-Earth Atmospheres. Astrophys. J. Lett. 2021;921:L28. doi: 10.3847/2041-8213/ac2f3a. [DOI] [Google Scholar]
- Eschenmoser A., Loewenthal E.. Chemistry of potentially prebiological natural products. Chem. Soc. Rev. 1992;21:1–16. doi: 10.1039/cs9922100001. [DOI] [Google Scholar]
- Balucani N.. Elementary reactions of N atoms with hydrocarbons: first steps towards the formation of prebiotic N-containing molecules in planetary atmospheres. Chem. Soc. Rev. 2012;41:5473–5483. doi: 10.1039/c2cs35113g. [DOI] [PubMed] [Google Scholar]
- Giani L., Ceccarelli C., Mancini L., Bianchi E., Pirani F., Rosi M., Balucani N.. Revised gas-phase formation network of methyl cyanide: the origin of methyl cyanide and methanol abundance correlation in hot corinos. Mon. Not. R. Astron. Soc. 2023;526:4535–4556. doi: 10.1093/mnras/stad2892. [DOI] [Google Scholar]
- Giani L., Bianchi E., Fournier M., Cheikh Sid Ely S., Ceccarelli C., Rosi M., Guillemin J.-C., Sims I. R., Balucani N.. A comprehensive study of the gas-phase formation network of HC5N: theory, experiments, observations, and models. Mon. Not. R. Astron. Soc. 2025;537:3861–3883. doi: 10.1093/mnras/staf189. [DOI] [Google Scholar]
- Loison J.-C., Wakelam V., Hickson K. M., Bergeat A., Mereau R.. The gas-phase chemistry of carbon chains in dark cloud chemical models. Mon. Not. R. Astron. Soc. 2013;437:930–945. doi: 10.1093/mnras/stt1956. [DOI] [Google Scholar]
- Balucani N., Asvany O., Huang L., Lee Y., Kaiser R., Osamura Y., Bettinger H.. Formation of Nitriles in the Interstellar Medium via Reactions ofCyano Radicals, CN (X2Σ+), withUnsaturated Hydrocarbons. Astrophy. J. 2000;545:892. doi: 10.1086/317848. [DOI] [Google Scholar]
- Le Gal R., Brady M. T., Öberg K. I., Roueff E., Le Petit F.. The Role of C/O in Nitrile Astrochemistry in PDRs and Planet-forming Disks. Astrophy. J. 2019;886:86. doi: 10.3847/1538-4357/ab4ad9. [DOI] [Google Scholar]
- Li X., Millar T. J., Walsh C., Heays A. N., van Dishoeck E. F.. Photodissociation and chemistry of N2 in the circumstellar envelope of carbon-rich AGB stars. Astron. Astrophys. 2014;568:A111. doi: 10.1051/0004-6361/201424076. [DOI] [Google Scholar]
- Cordiner M. A., Millar T. J.. Density-Enhanced Gas and Dust Shells in a New Chemical Model for IRC+10216. Astrophy. J. 2009;697:68–78. doi: 10.1088/0004-637X/697/1/68. [DOI] [Google Scholar]
- Cherchneff I., Glassgold A. E., Mamon G. A.. The Formation of Cyanopolyyne Molecules in IRC + 10216. Astrophy. J. 1993;410:188. doi: 10.1086/172737. [DOI] [Google Scholar]
- Yung Y. L., Allen M., Pinto J. P.. Photochemistry of the atmosphere of Titan - Comparison between model and observations. Astrophys. J. 1984;55:465–506. doi: 10.1086/190963. [DOI] [PubMed] [Google Scholar]
- Yung Y. L.. An update of nitrile photochemistry on Titan. Icarus. 1987;72:468–472. doi: 10.1016/0019-1035(87)90186-2. [DOI] [PubMed] [Google Scholar]
- Wilson E. H., Atreya S.. Current state of modeling the photochemistry of Titan’s mutually dependent atmosphere and ionosphere. J. Geophys. Res.: planets. 2004;109:E6. doi: 10.1029/2003JE002181. [DOI] [Google Scholar]
- Kaiser R. I., Balucani N.. The formation of nitriles in hydrocarbon-rich atmospheres of planets and their satellites: Laboratory investigations by the crossed molecular beam technique. Acc. Chem. Res. 2001;34:699–706. doi: 10.1021/ar000112v. [DOI] [PubMed] [Google Scholar]
- Balucani N., Asvany O., Osamura Y., Huang L., Lee Y., Kaiser R.. Laboratory investigation on the formation of unsaturated nitriles in Titan’s atmosphere. Planet. Space Sci. 2000;48:447–462. doi: 10.1016/S0032-0633(00)00018-0. [DOI] [Google Scholar]
- Lavvas P., Coustenis A., Vardavas I.. Coupling photochemistry with haze formation in Titan’s atmosphere, Part II: Results and validation with Cassini/Huygens data. Planet. Space Sci. 2008;56:67–99. doi: 10.1016/j.pss.2007.05.027. [DOI] [Google Scholar]
- Vuitton V., Yelle R., Klippenstein S., Hörst S., Lavvas P.. Simulating the density of organic species in the atmosphere of Titan with a coupled ion-neutral photochemical model. Icarus. 2019;324:120–197. doi: 10.1016/j.icarus.2018.06.013. [DOI] [Google Scholar]
- Loison J., Hébrard E., Dobrijevic M., Hickson K., Caralp F., Hue V., Gronoff G., Venot O., Bénilan Y.. The neutral photochemistry of nitriles, amines and imines in the atmosphere of Titan. Icarus. 2015;247:218–247. doi: 10.1016/j.icarus.2014.09.039. [DOI] [Google Scholar]
- Krasnopolsky V. A.. A photochemical model of Pluto’s atmosphere and ionosphere. Icarus. 2020;335:113374. doi: 10.1016/j.icarus.2019.07.008. [DOI] [Google Scholar]
- Krasnopol’Skii V.A., Tkachuk A.I., Korablev O.I.. C3 and CN parents in Comet P/Halley. Astron. Astrophys. 1991;245:310–315. [Google Scholar]
- Cordiner M., Charnley S.. Neutral–neutral synthesis of organic molecules in cometary comae. Mon. Not. R. Astron. Soc. 2021;504:5401–5408. doi: 10.1093/mnras/stab1123. [DOI] [Google Scholar]
- Ahmed S., Acharyya K.. The extent of formation of organic molecules in the comae of comets showing relatively high activity. Icarus. 2025;427:116374. doi: 10.1016/j.icarus.2024.116374. [DOI] [Google Scholar]
- Agúndez M., Bermúdez C., Cabezas C., Molpeceres G., Endo Y., Marcelino N., Tercero B., Guillemin J. C., de Vicente P., Cernicharo J.. The rich interstellar reservoir of dinitriles: Detection of malononitrile and maleonitrile in TMC-1. Astron. Astrophys. 2024;688:L31. doi: 10.1051/0004-6361/202451525. [DOI] [Google Scholar]
- Agúndez M., Marcelino N., Cernicharo J.. Discovery of Interstellar Isocyanogen (CNCN): Further Evidence that Dicyanopolyynes Are Abundant in Space. Astrophys. J. Lett. 2018;861:L22. doi: 10.3847/2041-8213/aad089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agúndez M.. et al. Probing non-polar interstellar molecules through their protonated form: Detection of protonated cyanogen (NCCNH+) Astron. Astrophys. 2015;579:L10. doi: 10.1051/0004-6361/201526650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas C., Bermúdez C., Gallego J. D., Tercero B., Hernández J. M., Tanarro I., Herrero V. J., Doménech J. L., Cernicharo J.. The millimeter-wave spectrum and astronomical search of succinonitrile and its vibrational excited states*. Astron. Astrophys. 2019;629:A35. doi: 10.1051/0004-6361/201935899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas C., Bermúdez C., Endo Y., Tercero B., Cernicharo J.. Rotational spectroscopy and astronomical search for glutaronitrile. Astron. Astrophys. 2020;636:A33. doi: 10.1051/0004-6361/202037769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitarra O., Lee K. L. K., Buchanan Z., Melosso M., McGuire B. A., Goubet M., Pirali O., Martin-Drumel M.-A.. Hunting the relatives of benzonitrile: Rotational spectroscopy of dicyanobenzenes*. Astron. Astrophys. 2021;652:A163. doi: 10.1051/0004-6361/202141386. [DOI] [Google Scholar]
- Agúndez M., Cabezas C., Marcelino N., Fuentetaja R., Tercero B., de Vicente P., Cernicharo J.. Discovery of interstellar NC4NH+: Dicyanopolyynes are indeed abundant in space. Astron. Astrophys. 2023;669:L1. doi: 10.1051/0004-6361/202245492. [DOI] [Google Scholar]
- Kołos R., Grabowski Z. R.. The chemistry and prospects for interstellar detection of somedicyanoacetylenes and other cyanoacetylene-related species. Astrophys. Space Sci. 2000;271:65–72. doi: 10.1023/A:1002183604278. [DOI] [Google Scholar]
- Petrie S., Millar T., Markwick A.. NCCN in TMC-1 and IRC+ 10216. Mon. Not. R. Astron. Soc. 2003;341:609–616. doi: 10.1046/j.1365-8711.2003.06436.x. [DOI] [Google Scholar]
- Subbaraman A., Kazi Z., Choughuley A., Chadha M.. Urea-acetylene dicarboxylic acid reaction: a likely pathway for prebiotic uracil formation. Orig. Life. 1980;10:343–347. doi: 10.1007/BF00928306. [DOI] [PubMed] [Google Scholar]
- Tsanakopoulou M., Xu J., Bond A. D., Sutherland J. D.. A new and potentially prebiotic α-cytidine derivative. Chem. Commun. 2017;53:3327–3329. doi: 10.1039/C7CC00693D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson R., Mayo L., Knuckles M., Khanna R.. C4N2 ice in Titan’s north polar stratosphere. Planet. Space Sci. 1997;45:941–948. doi: 10.1016/S0032-0633(97)00088-3. [DOI] [Google Scholar]
- Balucani N., Alagia M., Cartechini L., Casavecchia P., Volpi G. G., Sato K., Takayanagi T., Kurosaki Y.. Cyanomethylene formation from the reaction of excited nitrogen atoms with acetylene: a crossed beam and ab initio study. J. Am. Chem. Soc. 2000;122:4443–4450. doi: 10.1021/ja993448c. [DOI] [Google Scholar]
- Osamura Y., Petrie S.. NCCN and NCCCCN Formation in Titan’s Atmosphere: 1. Competing Reactions of Precursor HCCN(3A') with H(2S) and CH3(2A') J. Phys. Chem. A. 2004;108:3615–3622. doi: 10.1021/jp037817+. [DOI] [Google Scholar]
- Halpern J., Miller G., Okabe H.. The reaction of CN radicals with cyanoacetylene. Chem. Phys. Lett. 1989;155:347–350. doi: 10.1016/0009-2614(89)87167-2. [DOI] [Google Scholar]
- Faure A., Vuitton V., Thissen R., Wiesenfeld L.. A Semiempirical Capture Model for Fast Neutral Reactions at Low Temperature. J. Phys. Chem. A. 2009;113:13694–13699. doi: 10.1021/jp905609x. [DOI] [PubMed] [Google Scholar]
- Cheikh Sid Ely S., Morales S. B., Guillemin J.-C., Klippenstein S. J., Sims I. R.. Low Temperature Rate Coefficients for the Reaction CN + HC3N. J. Phys. Chem. A. 2013;117:12155–12164. doi: 10.1021/jp406842q. [DOI] [PubMed] [Google Scholar]
- Greenwald E. E., North S. W., Georgievskii Y., Klippenstein S. J.. A Two Transition State Model for Radical–Molecule Reactions: A Case Study of the Addition of OH to C2H4 . J. Phys. Chem. A. 2005;109:6031–6044. doi: 10.1021/jp058041a. [DOI] [PubMed] [Google Scholar]
- Valença Ferreira de Aragão E., Mancini L., Faginas-Lago N., Rosi M., Skouteris D., Pirani F.. Semiempirical Potential in Kinetics Calculations on the HC3N + CN Reaction. Molecules. 2022;27:2297. doi: 10.3390/molecules27072297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie S., Osamura Y.. NCCN and NCCCCN Formation in Titan’s Atmosphere: 2. HNC as a Viable Precursor. J. Phys. Chem. A. 2004;108:3623–3631. doi: 10.1021/jp0378182. [DOI] [Google Scholar]
- Balucani N., Leonori F., Petrucci R., Wang X., Casavecchia P., Skouteris D., Albernaz A. F., Gargano R.. A combined crossed molecular beams and theoretical study of the reaction CN+C2H4 . Chem. Phys. 2015;449:34–42. doi: 10.1016/j.chemphys.2014.12.014. [DOI] [Google Scholar]
- Leonori F., Petrucci R., Wang X., Casavecchia P., Balucani N.. A crossed beam study of the reaction CN+C2H4 at a high collision energy: The opening of a new reaction channel. Chem. Phys. Lett. 2012;553:1–5. doi: 10.1016/j.cplett.2012.09.070. [DOI] [Google Scholar]
- Casavecchia P., Balucani N., Cartechini L., Capozza G., Bergeat A., Volpi G. G.. Crossed beam studies of elementary reactions of N and C atoms and CN radicals of importance in combustion. Faraday Discuss. 2001;119:27–49. doi: 10.1039/b102634h. [DOI] [PubMed] [Google Scholar]
- Casavecchia P., Leonori F., Balucani N., Petrucci R., Capozza G., Segoloni E.. Probing the dynamics of polyatomic multichannel elementary reactions by crossed molecular beam experiments with soft electron-ionization mass spectrometric detection. Phys. Chem. Chem. Phys. 2009;11:46–65. doi: 10.1039/B814709D. [DOI] [PubMed] [Google Scholar]
- Casavecchia P., Leonori F., And N. B.. Reaction dynamics of oxygen atoms with unsaturated hydrocarbons from crossed molecular beam studies: primary products, branching ratios and role of intersystem crossing. Int. Rev. Phys. Chem. 2015;34:161–204. doi: 10.1080/0144235X.2015.1039293. [DOI] [Google Scholar]
- Daly N. R.. Scintillation Type Mass Spectrometer Ion Detector. Rev. Sci. Instrum. 1960;31:264–267. doi: 10.1063/1.1716953. [DOI] [Google Scholar]
- Alagia M., Aquilanti V., Ascenzi D., Balucani N., Cappelletti D., Cartechini L., Casavecchia P., Pirani F., Sanchini G., Volpi G. G.. Magnetic Analysis of Supersonic Beams of Atomic Oxygen, Nitrogen, and Chlorine Generated from a Radio-Frequency Discharge. Isr. J. Chem. 1997;37:329–342. doi: 10.1002/ijch.199700038. [DOI] [Google Scholar]
- Leonori F., Hickson K. M., Picard S. D. L., Wang X., Petrucci R., Foggi P., Balucani N., Casavecchia P.. Crossed-beam universal-detection reactive scattering of radical beams characterized by laser-induced-fluorescence: the case of C2 and CN. Mol. Phys. 2010;108:1097–1113. doi: 10.1080/00268971003657110. [DOI] [Google Scholar]
- Lagana A., Garcia E., Paladini A., Casavecchia P., Balucani N.. The last mile of molecular reaction dynamics virtual experiments: the case of the OH (N= 1–10)+ CO (j= 0–3) reaction. Faraday Discuss. 2012;157:415–436. doi: 10.1039/c2fd20046e. [DOI] [PubMed] [Google Scholar]
- Leonori F., Petrucci R., Balucani N., Hickson K. M., Hamberg M., Geppert W. D., Casavecchia P., Rosi M.. Crossed-Beam and Theoretical Studies of the S(1D) + C2H2 Reaction. J. Phys. Chem. A. 2009;113:4330–4339. doi: 10.1021/jp810989p. [DOI] [PubMed] [Google Scholar]
- Moureu C., Bongrand J. C.. Le cyanoacetylene C3NH. Ann. Chem. Paris. 1920;14:47–58. [Google Scholar]
- Liang P., Mancini L., Marchione D., Vanuzzo G., Ferlin F., Recio P., Tan Y., Pannacci G., Vaccaro L., Rosi M.. et al. Combined crossed molecular beams and computational study on the N(2D) + HCCCN(X1Σ+) reaction and implications for extra-terrestrial environments. Mol. Phys. 2022;120:e1948126. doi: 10.1080/00268976.2021.1948126. [DOI] [Google Scholar]
- Liang P., de Aragão E. V. F., Pannacci G., Vanuzzo G., Giustini A., Marchione D., Recio P., Ferlin F., Stranges D., Lago N. F., Rosi M., Casavecchia P., Balucani N.. Reactions O(3P, 1D) + HCCCN(X1Σ+) (Cyanoacetylene): Crossed-Beam and Theoretical Studies and Implications for the Chemistry of Extraterrestrial Environments. J. Phys. Chem. A. 2023;127:685–703. doi: 10.1021/acs.jpca.2c07708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch, M. , et al. Gaussian 09, Revision A. 02, 2009; Gaussian. Inc., Wallingford CT, 2009, 201. [Google Scholar]
- Leonori F., Petrucci R., Balucani N., Casavecchia P., Rosi M., Berteloite C., Le Picard S. D., Canosa A., Sims I. R.. Observation of organosulfur products (thiovinoxy, thioketene and thioformyl) in crossed-beam experiments and low temperature rate coefficients for the reaction S(1D) + C2H4 . Phys. Chem. Chem. Phys. 2009;11:4701–4706. doi: 10.1039/b900059c. [DOI] [PubMed] [Google Scholar]
- Berteloite C., Le Picard S. D., Sims I. R., Rosi M., Leonori F., Petrucci R., Balucani N., Wang X., Casavecchia P.. Low temperature kinetics, crossed beam dynamics and theoretical studies of the reaction S(1D)+ CH4 and low temperature kinetics of S(1D)+ C2H2 . Phys. Chem. Chem. Phys. 2011;13:8485–8501. doi: 10.1039/c0cp02813d. [DOI] [PubMed] [Google Scholar]
- Leonori F., Skouteris D., Petrucci R., Casavecchia P., Rosi M., Balucani N.. Combined crossed beam and theoretical studies of the C(1D)+ CH4 reaction. J. Chem. Phys. 2013;138:024311. doi: 10.1063/1.4773579. [DOI] [PubMed] [Google Scholar]
- Valença Ferreira de Aragão, E. ; Faginas-Lago, N. ; Rosi, M. ; Mancini, L. ; Balucani, N. ; Skouteris, D. ; A Computational Study of the Reaction Cyanoacetylene and Cyano Radical Leading to 2-Butynedinitrile and Hydrogen Radical. Computational Science and Its Applications – ICCSA 2020.; Springer; Cham, 2020. pp. 707–716. [Google Scholar]
- Becke A. D.. Density Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993;98:5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- Stephens P. J., Devlin F. J., Chabalowski C. F., Frisch M. J.. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994;98:11623–11627. doi: 10.1021/j100096a001. [DOI] [Google Scholar]
- Dunning Jr T. H.. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989;90:1007–1023. doi: 10.1063/1.456153. [DOI] [Google Scholar]
- Zhao Y., Truhlar D. G.. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008;120:215–241. doi: 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- McLean A. D., Chandler G. S.. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980;72:5639–5648. doi: 10.1063/1.438980. [DOI] [Google Scholar]
- Krishnan R. B. J. S., Binkley J.S., Seeger R., Pople J.A.. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980;72:650–654. doi: 10.1063/1.438955. [DOI] [Google Scholar]
- Gonzalez C., Schlegel H. B.. An improved algorithm for reaction path following. J. Chem. Phys. 1989;90:2154–2161. doi: 10.1063/1.456010. [DOI] [Google Scholar]
- Gonzalez C., Schlegel H. B.. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990;94:5523–5527. doi: 10.1021/j100377a021. [DOI] [Google Scholar]
- Bartlett R. J.. Many-body perturbation theory and coupled cluster theory for electron correlation in molecules. Annu. Rev. Phys. Chem. 1981;32:359–401. doi: 10.1146/annurev.pc.32.100181.002043. [DOI] [Google Scholar]
- Raghavachari K., Trucks G. W., Pople J. A., Head-Gordon M.. A fifth-order perturbation comparison of electron correlation theories. Chem. Phys. Lett. 1989;157:479–483. doi: 10.1016/S0009-2614(89)87395-6. [DOI] [Google Scholar]
- Olsen J., Jørgensen P., Koch H., Balkova A., Bartlett R. J.. Full configuration–interaction and state of the art correlation calculations on water in a valence double-zeta basis with polarization functions. J. Chem. Phys. 1996;104:8007–8015. doi: 10.1063/1.471518. [DOI] [Google Scholar]
- Lee T. J., Taylor P. R.. A diagnostic for determining the quality of single-reference electron correlation methods. Int. J. Quantum Chem. 1989;36:199–207. doi: 10.1002/qua.560360824. [DOI] [Google Scholar]
- Martin J. M.. Ab initio total atomization energies of small molecules–towards the basis set limit. Chem. Phys. Lett. 1996;259:669–678. doi: 10.1016/0009-2614(96)00898-6. [DOI] [Google Scholar]
- Balucani N., Capozza G., Leonori F., Segoloni E., Casavecchia P.. Crossed molecular beam reactive scattering: from simple triatomic to multichannel polyatomic reactions. Int. Rev. Phys. Chem. 2006;25:109–163. doi: 10.1080/01442350600641305. [DOI] [Google Scholar]
- Olkhov R. V., Smith I. W. M.. Rate coefficients for reaction and for rotational energy transfer in collisions between CN in selected rotational levels (X 2Σ+, v = 2, N = , 1, 6, 10, 15, and 20) and C2H2 . J. Chem. Phys. 2007;126:134314. doi: 10.1063/1.2715594. [DOI] [PubMed] [Google Scholar]
- Herbert L., Smith I. W. M., Spencer-Smith R. D.. Rate constants for the elementary reactions between CN radicals and CH4, C2H6, C2H4, C3H6, and C2H2 in the range: 295 ≤ T/K ≤ 700. Int. J. Chem. Kinet. 1992;24:791–802. doi: 10.1002/kin.550240904. [DOI] [Google Scholar]
- Huang L. C. L., Asvany O., Chang A. H. H., Balucani N., Lin S. H., Lee Y. T., Kaiser R. I., Osamura Y.. Crossed beam reaction of cyano radicals with hydrocarbon molecules. IV. Chemical dynamics of cyanoacetylene (HCCCN; X 1 Σ +) formation from reaction of CN(X 2 Σ +) with acetylene, C2H2 . J. Chem. Phys. 2000;113:8656–8666. doi: 10.1063/1.1289530. [DOI] [Google Scholar]
- Balucani N., Asvany O., Chang A., Lin S., Lee Y., Kaiser R., Osamura Y.. Crossed beam reaction of cyano radicals with hydrocarbon molecules. III. Chemical dynamics of vinylcyanide (C2H3CN; X 1 A’) formation from reaction of CN(X 2Σ+) with ethylene, C2H4 (X 1 A g ) J. Chem. Phys. 2000;113:8643–8655. doi: 10.1063/1.1289529. [DOI] [Google Scholar]
- Marchione D., Mancini L., Liang P., Vanuzzo G., Pirani F., Skouteris D., Rosi M., Casavecchia P., Balucani N.. Unsaturated dinitriles formation routes in extraterrestrial environments: A combined experimental and theoretical investigation of the reaction between cyano radicals and cyanoethene (C2H3CN) J. Phys. Chem. A. 2022;126:3569–3582. doi: 10.1021/acs.jpca.2c01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balucani N., Bergeat A., Cartechini L., Volpi G. G., Casavecchia P., Skouteris D., Rosi M.. Combined crossed molecular beam and theoretical studies of the N (2D)+ CH4 reaction and implications for atmospheric models of Titan. J. Phys. Chem. A. 2009;113:11138–11152. doi: 10.1021/jp904302g. [DOI] [PubMed] [Google Scholar]
- Homayoon Z., Bowman J. M., Balucani N., Casavecchia P.. Quasiclassical trajectory calculations of the N (2D)+ H2O reaction elucidating the formation mechanism of HNO and HON seen in molecular beam experiments. J. Phys. Chem. Lett. 2014;5:3508–3513. doi: 10.1021/jz501757s. [DOI] [PubMed] [Google Scholar]
- Yang D. L., Yu T., Lin M. C., Melius C. F.. CN radical reactions with hydrogen cyanide and cyanogen: Comparison of theory and experiment. J. Chem. Phys. 1992;97:222–226. doi: 10.1063/1.463945. [DOI] [Google Scholar]
- Wooldridge S. T., Hanson R. K., Bowman C. T.. A shock tube study of reactions of CN with HCN, OH, and H2 using CN and OH laser absorption. Int. J. Chem. Kinet. 1996;28:245–258. doi: 10.1002/(SICI)1097-4601(1996)28:4<245::AID-KIN2>3.0.CO;2-V. [DOI] [Google Scholar]
- Vanuzzo G., Marchione D., Mancini L., Liang P., Pannacci G., Recio P., Tan Y., Rosi M., Skouteris D., Casavecchia P.. et al. The N (2D)+ CH2CHCN (vinyl cyanide) reaction: a combined crossed molecular beam and theoretical study and implications for the atmosphere of titan. J. Phys. Chem. A. 2022;126:6110–6123. doi: 10.1021/acs.jpca.2c04263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini L., Rosi M., Skouteris D., Vanuzzo G., Pannacci G., Casavecchia P., Balucani N.. A computational characterization of the reaction mechanisms for the reactions N (2D)+ CH3CN and HC3N and implications for the nitrogen-rich organic chemistry of Titan. Comput. Theor. Chem. 2023;1229:114341. doi: 10.1016/j.comptc.2023.114341. [DOI] [Google Scholar]
- Nguyen T. L., Vereecken L., Hou X. J., Nguyen M. T., Peeters J.. Potential energy surfaces, product distributions and thermal rate coefficients of the reaction of O (3P) with C2H4 (X Ag): a comprehensive theoretical study. J. Phys. Chem. A. 2005;109:7489–7499. doi: 10.1021/jp052970k. [DOI] [PubMed] [Google Scholar]
- Recio P., Alessandrini S., Vanuzzo G., Pannacci G., Baggioli A., Marchione D., Caracciolo A., Murray V. J., Casavecchia P., Balucani N.. et al. Intersystem crossing in the entrance channel of the reaction of O (3P) with pyridine. Nat. Chem. 2022;14:1405–1412. doi: 10.1038/s41557-022-01047-3. [DOI] [PubMed] [Google Scholar]
- Loomis R. A., Burkhardt A. M., Shingledecker C. N., Charnley S. B., Cordiner M. A., Herbst E., Kalenskii S., Lee K. L. K., Willis E. R., Xue C., Remijan A. J., McCarthy M. C., McGuire B. A.. An investigation of spectral line stacking techniques and application to the detection of HC11N. Nat. Astron. 2021;5:188–196. doi: 10.1038/s41550-020-01261-4. [DOI] [Google Scholar]
- Seki K., Yagi M., He M., Halpern J. B., Okabe H.. Reaction rates of the CN radical with diacetylene and dicyanoacetylene. Chem. Phys. Lett. 1996;258:657–662. doi: 10.1016/0009-2614(96)00697-5. [DOI] [Google Scholar]
- Cernicharo J., Marcelino N., Agúndez M., Bermúdez C., Cabezas C., Tercero B., Pardo J. R.. Discovery of HC4NC in TMC-1: A study of the isomers of HC3N, HC5N, and HC7N. Astron. Astrophys. 2020;642:L8. doi: 10.1051/0004-6361/202039274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C., Willis E. R., Loomis R. A., Kelvin Lee K. L., Burkhardt A. M., Shingledecker C. N., Charnley S. B., Cordiner M. A., Kalenskii S., McCarthy M. C., Herbst E., Remijan A. J., McGuire B. A.. Detection of Interstellar HC4NC and an Investigation of Isocyanopolyyne Chemistry under TMC-1 Conditions. Astrophys. J. Lett. 2020;900:L9. doi: 10.3847/2041-8213/aba631. [DOI] [Google Scholar]
- Li H. Y., Cheng W. C., Liu Y. L., Sun B. J., Huang C. Y., Chen K. T., Tang M. S., Kaiser R. I., Chang A. H. H.. Reaction of cyanoacetylene HCCCN(X 1Σ+) with ground-state carbon atoms C(3P) in cold molecular clouds. J. Chem. Phys. 2006;124:044307. doi: 10.1063/1.2148411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








