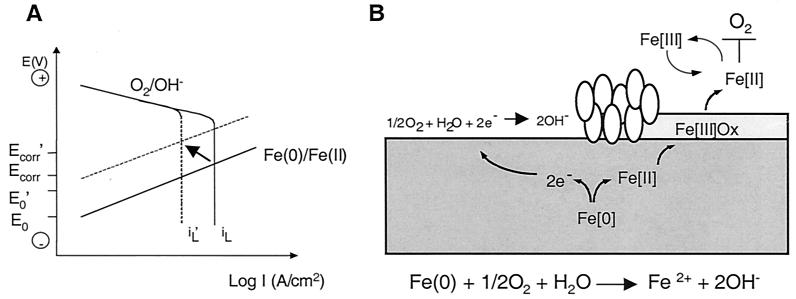
FIG. 4.
Model for corrosion protection based on microbial respiration of oxygen and iron. (A) Theoretical polarization curves for corrosion of iron in a neutral, aerated solution. The straight lines correspond to the iron oxidation reaction that is under charge transfer control. The polarization curves for oxygen reduction consist of a charge transfer-controlled reaction at low current densities, followed by a limiting current density (iL) for the reduction of oxygen, which is under mass transport control. The Ecorr and the corrosion current density (icorr) are located at the intersection of the polarization curves for the oxidation and reduction reactions. The arrow indicates the direction of the expected shift in the curves in a system with wild-type S. oneidensis. (B) Diagram showing how oxygen consumption that is mediated by direct microbial oxygen respiration and indirect reduction by ferrous iron (produced by ferric iron respiration) protects steel from corrosion. The general equation for corrosion is given. The ovals represent cells that are actively respiring at the steel surface. Ferrous iron produced by respiration of Fe(III) oxides forms a reducing shield that blocks oxygen from attacking the steel surface.