Abstract
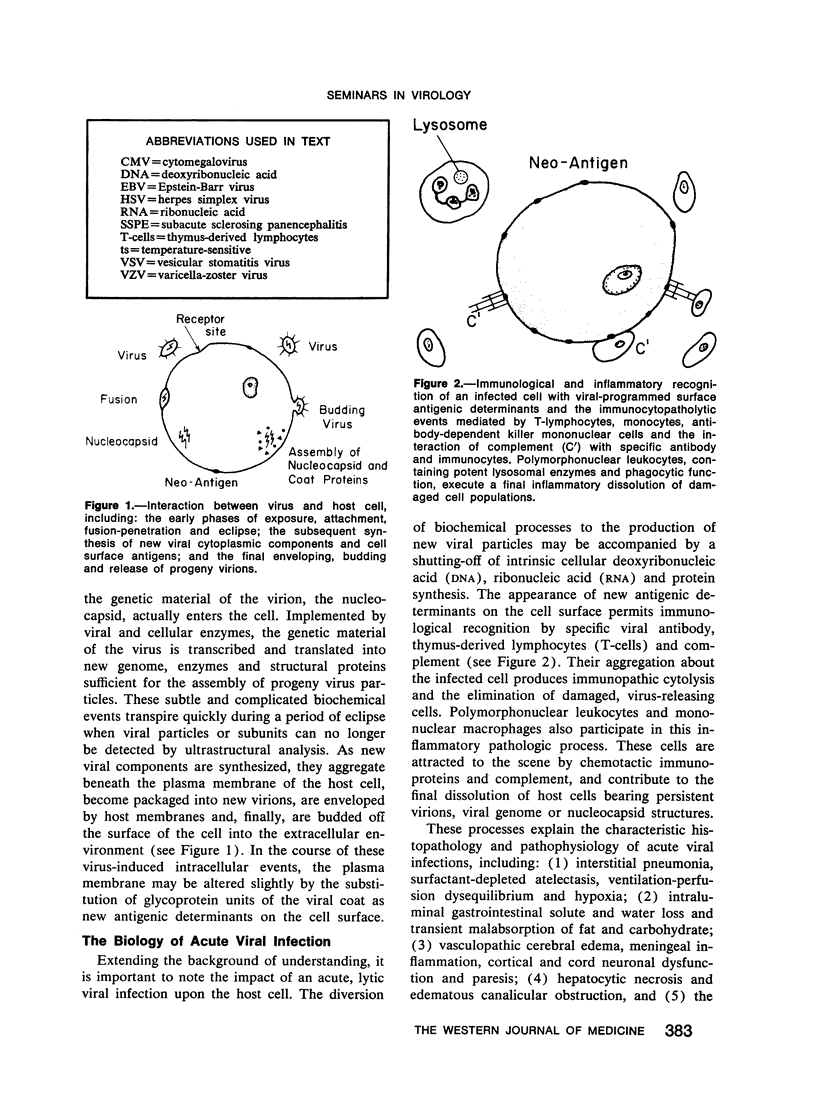
Sequential events characterize the interaction of viruses with parenchymal cells, and acute lytic infections of tissues and organs have broad biological attributes. A knowledge of these permits a keener understanding of persistent, intermittent herpesvirus infections and persistent, continuous respiratory virus infections. In addition to unique biochemical mechanisms which may permit the latter chronic infections to evolve, the roles of defective and mutant strains of virus, viral interference, and the genetic, developmental and immunological expressions of the host are of considerable and provocative importance.
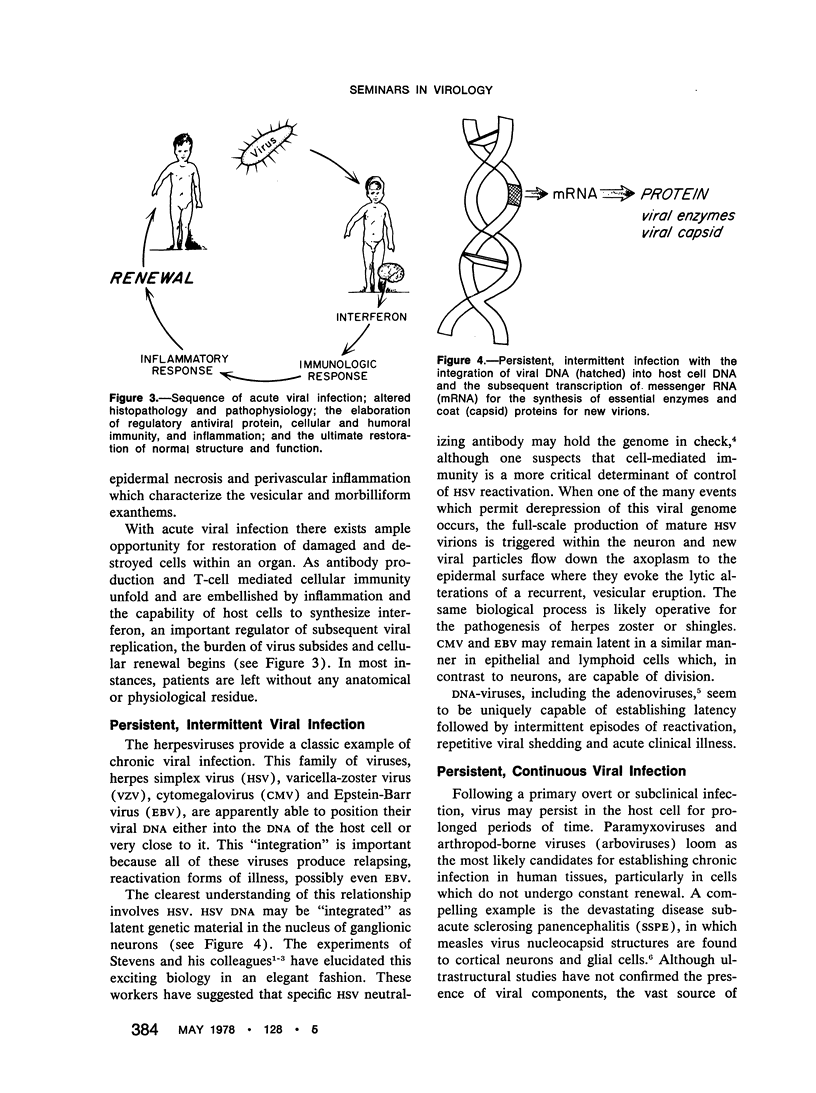
The traditional view of viral infections embraces a broad spectrum of acute pathological and inflammatory events. The relationship of measles virus to subacute sclerosing panencephalitis, the elucidation of the latency of herpes simplex virus, and the slow unmasking of the pathogenesis of multiple sclerosis have illustrated the subtle elements of persistent viral infections of the human being. These chronic neurological diseases have provided the opportunity and stimulus for sharp dissection of the biological and biochemical processes which embellish the logical link of viral infections to other forms of chronic human illness.
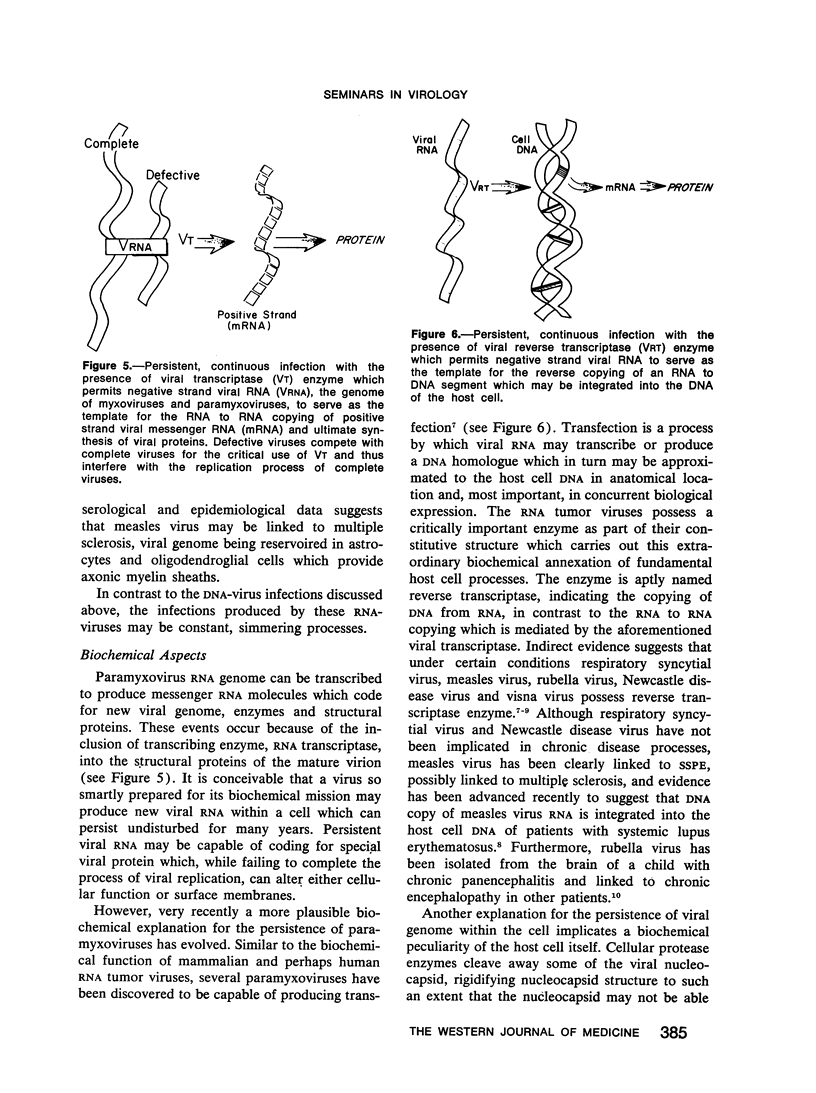
Full text
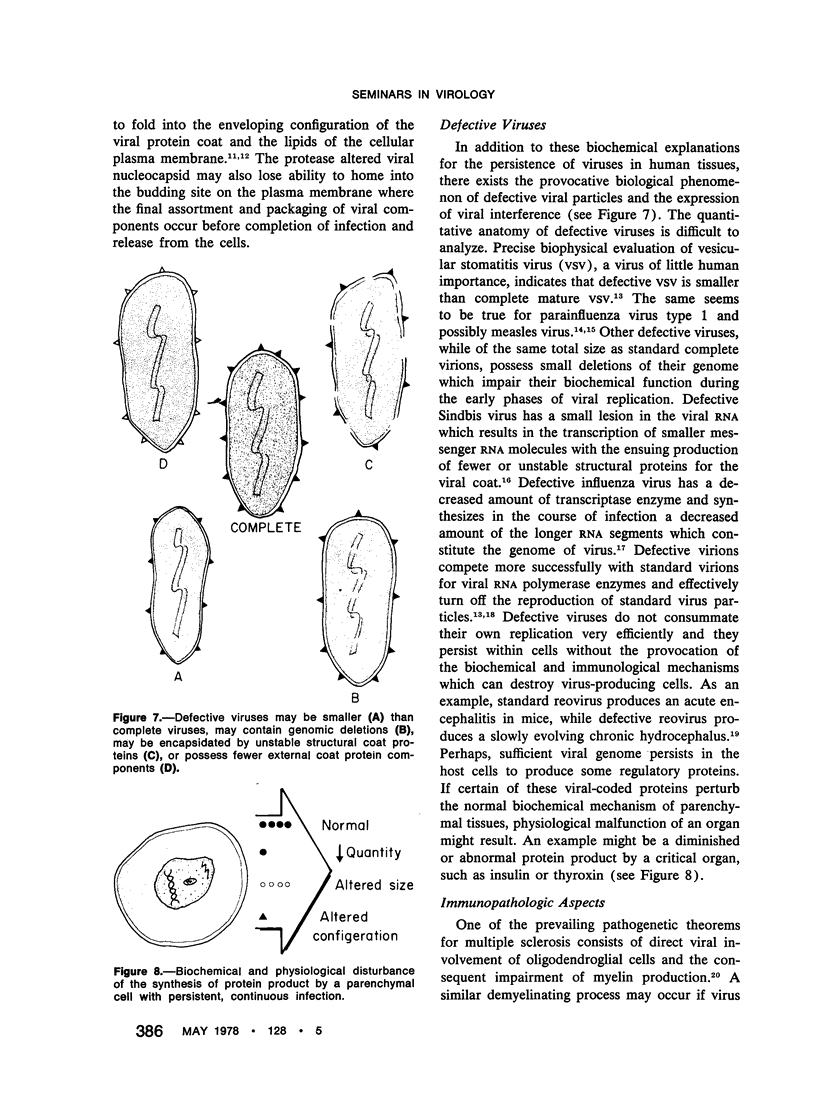
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Dickinson J. P. Aetiology of multiple sclerosis. Lancet. 1974 Jun 15;1(7868):1196–1199. doi: 10.1016/s0140-6736(74)91005-8. [DOI] [PubMed] [Google Scholar]
- Albrecht P., Burnstein T., Klutch M. J., Hicks H. T., Ennis F. A. Subacute sclerosing panencephalitis: experimental infection in primates. Science. 1977 Jan 7;195(4273):64–66. doi: 10.1126/science.831255. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Transcriptase activity and genome composition of defective influenza virus. J Virol. 1976 Apr;18(1):365–369. doi: 10.1128/jvi.18.1.365-369.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholz C. M., Kiley M. P., Payne F. E. Isolation and characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Jul;16(1):192–202. doi: 10.1128/jvi.16.1.192-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher D. W., Notkins A. L. Virus-induced diabetes mellitus. I. Hyperglycemia and hypoinsulinemia in mice infected with encephalomyocarditis virus. J Exp Med. 1973 May 1;137(5):1226–1239. doi: 10.1084/jem.137.5.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschkin A. M., Haspel M. V., Rapp F. Neurovirulence and induction of hydrocephalus with parental, mutant, and revertant strains of measles virus. J Virol. 1976 May;18(2):809–811. doi: 10.1128/jvi.18.2.809-811.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byington D. P., Johnson K. P. Experimental subacute sclerosing panencephalitis in the hamster: correlation of age with chronic inclusion-cell encephalitis. J Infect Dis. 1972 Jul;126(1):18–26. doi: 10.1093/infdis/126.1.18. [DOI] [PubMed] [Google Scholar]
- Ciongoli A. K., Platz P., Dupont B., Svejgaard A., Fog T., Jersild C. Letter: Lack of antigen response to myxoviruses in multiple sclerosis. Lancet. 1973 Nov 17;2(7838):1147–1147. [PubMed] [Google Scholar]
- Cook M. L., Bastone V. B., Stevens J. G. Evidence that neurons harbor latent herpes simplex virus. Infect Immun. 1974 May;9(5):946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Latent herpetic infections following experimental viraemia. J Gen Virol. 1976 Apr;31(1):75–80. doi: 10.1099/0022-1317-31-1-75. [DOI] [PubMed] [Google Scholar]
- Cremer N. E., Oshiro L. S., Weil M. L., Lennette E. H., Itabashi H. H., Carnay L. Isolation of rubella virus from brain in chronic progressive panencephalitis. J Gen Virol. 1975 Nov;29(2):143–153. doi: 10.1099/0022-1317-29-2-143. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C. Status spongiousus resulting from intracerebral infection of mice with temperature-sensitive mutants of vesicular stomatitis virus. Br J Exp Pathol. 1976 Jun;57(3):321–330. [PMC free article] [PubMed] [Google Scholar]
- Darnell M. B., Koprowski H. Genetically determined resistance to infection with group B arboviruses. II. Increased production of interfering particles in cell cultures from resistant mice. J Infect Dis. 1974 Mar;129(3):248–256. doi: 10.1093/infdis/129.3.248. [DOI] [PubMed] [Google Scholar]
- Fields B. N. Genetic manipulation of reovirus--a model for modification of disease. N Engl J Med. 1972 Nov 16;287(20):1026–1033. doi: 10.1056/NEJM197211162872007. [DOI] [PubMed] [Google Scholar]
- From the National Institutes of Health: current understanding of persistent viral infections and their implications in human disease--summary of a workshop. J Infect Dis. 1976 Jun;133(6):707–714. doi: 10.1093/infdis/133.6.707. [DOI] [PubMed] [Google Scholar]
- Furman P. A., Hallum J. V. RNA-dependent DNA polymerase activity in preparations of a mutant of Newcastle disease virus arising from persistently infected L cells. J Virol. 1973 Sep;12(3):548–555. doi: 10.1128/jvi.12.3.548-555.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble D. R., Taylor K. W., Cumming H. Coxsackie viruses and diabetes mellitus. Br Med J. 1973 Nov 3;4(5887):260–262. doi: 10.1136/bmj.4.5887.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi J. D., Sharp D. G. Host-induced influences on the syntheses of defective vaccinia particles. Virology. 1976 Aug;73(1):165–172. doi: 10.1016/0042-6822(76)90070-2. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Narayan P., Griffin D., Price D. Slow persistent infection caused by visna virus: role of host restriction. Science. 1977 Jan 14;195(4274):175–177. doi: 10.1126/science.188133. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Farris W. A. Follow-up of children infected in a coxsackievirus B-3 and B-4 outbreak: no evidence of diabetes mellitus. J Infect Dis. 1974 Jun;129(6):741–746. doi: 10.1093/infdis/129.6.741. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Purification of defective interfering T particles of vesicular stomatitis and rabies viruses generated in vivo in brains of newborn mice. Virology. 1975 Oct;67(2):438–449. doi: 10.1016/0042-6822(75)90445-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Perrin L. H., Oldstone M. B. Measurement of virus antigens on the surface of HeLa cells persistently infected with wild type and vaccine strains of measles virus by radioimmune assay. J Gen Virol. 1976 Mar;30(3):329–337. doi: 10.1099/0022-1317-30-3-329. [DOI] [PubMed] [Google Scholar]
- Kiley M. P., Gray R. H., Payne F. E. Replication of measles virus: distinct species of short nucleocapsids in cytoplasmic extracts of infected cells. J Virol. 1974 Mar;13(3):721–728. doi: 10.1128/jvi.13.3.721-728.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Olsen R., Confer A., Koestner A., McCullough B. Serologic response to canine distemper viral antigens in gnotobiotic dogs infected with canine distemper virus. J Infect Dis. 1975 Oct;132(4):384–392. doi: 10.1093/infdis/132.4.384. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Joseph B. S., Oldstone M. B. Antibody-induced capping of measles virus antigens on plasma membrane studied by electron microscopy. J Virol. 1975 May;15(5):1248–1255. doi: 10.1128/jvi.15.5.1248-1255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. L., Auerbach P. S., Hayes E. C. A blood test for multiple sclerosis based on the adherence of lymphocytes to measles-infected cells. N Engl J Med. 1976 Jun 24;294(26):1423–1427. doi: 10.1056/NEJM197606242942604. [DOI] [PubMed] [Google Scholar]
- McLaren L. C., Holland J. J. Defective interfering particles from poliovirus vaccine and vaccine reference strains. Virology. 1974 Aug;60(2):579–583. doi: 10.1016/0042-6822(74)90352-3. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Lackland H., Choppin P. W. Proteolytic cleavage of subunits of the nucleocapsid of the paramyxovirus simian virus 5. J Virol. 1974 Nov;14(5):1253–1261. doi: 10.1128/jvi.14.5.1253-1261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Holland J. Variability of vesicular stomatitis virus autointerference with different host cells and virus serotypes. Virology. 1972 Oct;50(1):148–158. doi: 10.1016/0042-6822(72)90355-8. [DOI] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Marx P. S., Kingsbury D. W. A temperature-sensitive mutant of Sendai virus with an altered hemagglutinin-neuraminidase polypeptide: consequences for virus assembly and cytopathology. Virology. 1975 Sep;67(1):179–187. doi: 10.1016/0042-6822(75)90415-8. [DOI] [PubMed] [Google Scholar]
- Roman J. M., Simon E. H. Defective interfering particles in monolayer-propagated Newcastle disease virus. Virology. 1976 Jan;69(1):298–303. doi: 10.1016/0042-6822(76)90216-6. [DOI] [PubMed] [Google Scholar]
- Schick J., Baczko K., Fanning E., Groneberg J., Burger H., Doerfler W. Intracellular forms of adenovirus DNA: integrated form of adenovirus DNA appears early in productive infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1043–1047. doi: 10.1073/pnas.73.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Iinuma M. Recovery of infectious proviral DNA from mammalian cells infected with respiratory syncytial virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3230–3234. doi: 10.1073/pnas.72.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffer D., Rannon L., Alter M., Kahana E., Feldman S. Subacute sclerosing panencephalitis: an epidemiologic study in Israel. Am J Epidemiol. 1976 Jan;103(1):67–74. doi: 10.1093/oxfordjournals.aje.a112206. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Maintenance of latent herpetic infection: an apparent role for anti-viral IgG. J Immunol. 1974 Dec;113(6):1685–1693. [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Lucas S. J., Albrecht P. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975 Sep 1;142(3):773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultz H. A., Hart B. A., Zielezny M., Schlesinger E. R. Is mumps virus an etiologic factor in juvenile diabetes mellitus. J Pediatr. 1975 Apr;86(4):654–656. doi: 10.1016/s0022-3476(75)80206-x. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Park M. S., Opelz G., Ting A. Multiple sclerosis and high incidence of a B lymphocyte antigen. Science. 1976 Sep 24;193(4259):1245–1247. doi: 10.1126/science.1085490. [DOI] [PubMed] [Google Scholar]
- Utermohlen V., Zabriskie J. B. A suppression of cellular immunity in patients with multiple sclerosis. J Exp Med. 1973 Dec 1;138(6):1591–1596. doi: 10.1084/jem.138.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters D. J., Bussell R. H. Isolation and comparative study of the nucleocapsids of measles and canine distemper viruses from infected cells. Virology. 1974 Sep;61(1):74–79. [PubMed] [Google Scholar]
- Weiner L. P., Johnson R. T., Herndon R. M. Viral infections and demyelinating diseases. N Engl J Med. 1973 May 24;288(21):1103–1110. doi: 10.1056/NEJM197305242882106. [DOI] [PubMed] [Google Scholar]
- Weiss B., Goran D., Cancedda R., Schlesinger S. Defective interfering passages of Sindbis virus: nature of the intracellular defective viral RNA. J Virol. 1974 Nov;14(5):1189–1198. doi: 10.1128/jvi.14.5.1189-1198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


