Abstract
The viability of bacteria in milk after heat treatments was assessed by using three different viability indicators: (i) CFU on plate count agar, (ii) de novo expression of a gfp reporter gene, and (iii) membrane integrity based on propidium iodide exclusion. In commercially available pasteurized milk, direct viable counts, based on dye exclusion, were significantly (P < 0.05) higher than viable cell counts determined from CFU, suggesting that a significant subpopulation of cells in pasteurized milk are viable but nonculturable. Heating milk at 63.5°C for 30 min resulted in a >4-log-unit reduction in the number of CFU of Escherichia coli and Pseudomonas putida that were marked with lac-inducible gfp. However, the reduction in the number of gfp-expressing cells of both organisms under the same conditions was <2.5 log units. These results demonstrate that a substantial portion of cells rendered incapable of forming colonies by heat treatment are metabolically active and are able to transcribe and translate genes de novo.
Milk pasteurization was introduced as a public health measure in order to destroy human pathogens and eliminate or reduce the activities of spoilage microorganisms. Bacillus spp. and Clostridium spp. are the organisms most likely to survive pasteurization as a consequence of their ability to form heat-resistant endospores. However, non-endospore-forming bacteria, including the pathogens Mycobacterium paratuberculosis and Listeria monocytogenes, can also survive pasteurization (3, 12). Sung and Collins (25) have demonstrated that due to its high D value (decimal reduction time), M. paratuberculosis may not be eliminated by high-temperature, short-time (HTST) pasteurization when initial concentrations are ≥101 cells ml−1. Binderova and Rysanek (3) have noted survival of L. monocytogenes and Escherichia coli serotype O157:H7 after HTST pasteurization. Survival of spoilage microorganisms, or their enzymes, after pasteurization is also important to spoilage of processed milk products. From a milk spoilage perspective, psychrotrophic microorganisms are the most important group of microbes present in milk and dairy products. Pseudomonas spp. are considered the most important psychrotrophs contributing to milk spoilage through production of lipolytic and proteolytic enzymes (29).
Culture-based techniques are most commonly used to determine viable counts in dairy products, but they have the limitation that they are unable to enumerate viable but nonculturable (VBNC) organisms (2, 9). The term VBNC is often used to describe cells that are metabolically active but cannot be cultured (20, 22). The VBNC state can be a survival mechanism adopted by many bacteria when exposed to adverse environmental conditions (2). Alternatively, VBNC organisms may be injured bacteria that have lost the ability to grow on laboratory media. VBNC organisms may potentially be capable of causing infections and may contribute to milk spoilage.
Direct analysis of microbial cells based on vital staining and combined with analysis by microscopy or flow cytometry can provide an alternative approach to culture-based methods for determining the total and viable counts of bacteria (28). A wide range of vital stains are now available (Hand Book of Fluorescent Probes and Research Chemicals, 6th ed., Molecular Probes, Inc., Eugene, Oreg.); most are based on various indicators of cell viability such as membrane potential, membrane integrity, or viability-linked enzymes (9, 19). Such direct approaches have the advantages that they do not depend on culturing of samples and that they can provide data on the physiological status of individual cells in a population in real time. However, results obtained by use of direct methods can be difficult to interpret if compared to standard culture-based methods. Such stains have been used to assess the viability of bacteria in milk and dairy products (1).
Gene expression using inducible genetic markers offers an alternative approach for assessing viability in both culturable and nonculturable cells. The green fluorescent protein (GFP) from the jellyfish (Aequorea victoria) (26) is particularly useful for such studies because it enables gene expression in individual cells to be examined nondestructively and in real time by using fluorescence microscopy or flow cytometry. GFP gene fusions have been used to enumerate and determine the spatial location of culturable and VBNC bacteria (5, 6) and to study the viability of starved bacteria (20), the viability of bacteria in environmental samples and rhizospheres (5, 23), and bacterial VBNC states (6, 20).
Here we describe the use of an inducible reporter gene expression system based on GFP to examine de novo gene expression in individual cells of VBNC bacteria after pasteurization. Additionally, a fluorescent dye exclusion assay was used to assess the viability of individual cells. This was achieved by using the LIVE/DEAD BacLight kit (Molecular Probes Inc.), which differentiates between live and dead bacteria based on membrane integrity. The kit relies on staining of total bacteria with a green fluorescent dye (SYTO9) and staining of dead or damaged bacteria by using propidium iodide (PI) (orange-red fluorescence).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth condition.
Bacterial strains and plasmids are listed in Table 1. Triparental matings were carried out to transfer GFP constructs to other bacteria as described by Hansen et al. (14). Donor strains used in triparental filter matings are shown in Table 1. E. coli strain CSH26::lacIq1/pKJK10 and Pseudomonas putida strain KT2442::lacIq1/pKJK10 carry the gfp structural gene under the control of the inducible lac promoter. E. coli CSH26::lacIq1/pKJK10 and P. putida KT2442::lacIq1/pKJK10 were used to inoculate ultra-heat-treated (UHT) milk. Bacteria were grown in Luria-Bertani (LB) medium at 37°C on a rotary shaker (180 rpm) for 16 h, and cells were diluted in phosphate-buffered saline (PBS) before inoculation of UHT milk.
TABLE 1.
Strains and plasmids used in this study
| Strain/plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| E. coli CSH26::lacIq1/pKJK10 | Tcr Smr Kmr | S. Sørensen |
| P. putida KT2442::lacIq1/pKJK10 | Tcr Smr Kmr | Sengeløv et al. (24) |
| E. coli MT102::pir/pUT Apr::lacIq Kmr | Apr Kmr | S. Sørensen |
| E. coli NF1815/pRK600 | Cmr | Strain collection (Macquarie University) |
| E. coli DH5α/pAGM42 | Smr Kmr | Sengeløv et al. (24) |
Tcr, tetracycline resistance; Apr, ampicillin resistance; Kmr, kanamycin resistance; Smr, streptomycin resistance.
Gene expression by bacteria in pasteurized milk.
UHT milk was inoculated with GFP-marked E. coli (2.5 × 106 ml−1) and P. putida (5.8 × 105 ml−1) and was pasteurized (see below). GFP expression was induced by addition of 5 to 10 mmol of isopropyl-β-d-thiogalactopyranoside (IPTG) in the presence of LB medium for 2 h at 37 and 30°C for E. coli and P. putida, respectively. The antibiotic nalidixic acid sodium salt (100 μg ml−1) was added to prevent DNA replication and cell division (18) by cells analyzed for GFP. Microscopic analysis revealed that the nalidixic acid treatment led to formation of long-form morphology by all cells (data not shown), indicating that the conditions did prevent replication and division. Nalidixic acid was not added for the subsamples used for the plate count method.
Milk samples and pasteurization treatments.
Either pasteurized-milk samples were obtained directly from an HTST (72°C for 15 s) commercial pasteurization unit of a dairy plant in Sydney, or milk was pasteurized in the laboratory by the test tube holder method (12): Briefly, 5 ml of UHT milk was dispensed into sterile glass tubes (13 by 100 mm). Tubes were placed in a water bath set at 63.5°C with the water level several inches above the level of the milk in the tubes. As a control, one tube contained a thermometer submerged in the milk. When the milk reached 63.5°C, experimental tubes were inoculated with bacteria and held for 30 min. After pasteurization, tubes were transferred to ice for rapid cooling. To establish the efficiency of the pasteurization treatment, a sample of raw milk was subjected to the same heating process and then assayed for phosphatase activity (17) to check that efficient heating had occurred (data not shown).
Determination of CFU.
Plate count methods were used to determine culturable cell numbers. Suspensions of E. coli or P. putida in milk were serially diluted in PBS, and 100-μl volumes of dilutions were spread-plated in triplicate. To select and differentiate E. coli, Chromocult coliform agar (CCA; Merck Pty Ltd., Sydney, Australia) was used, whereas Pseudomonas selective agar (PSA; Oxoid Pty. Ltd., Heidelberg, Victoria, Australia) was used to select P. putida. Colonies were counted after incubation of plates for 72 h at 30°C. Total viable microbial counts of raw milk and pasteurized milk were determined by standard plate count using the pour plate method as described by Desmasures and Gueguen (10). Samples were serially diluted in PBS, and appropriate dilutions were added to plate count agar (PCA), which contained the following, in grams per liter: tryptone, 5; yeast extract, 2.5; d-glucose, 1; bacteriological agar, 12. Colonies were counted after incubation of PCA plates for 72 h at 30°C.
Flow cytometry.
Samples were analyzed by using a FACScalibur flow cytometer (Becton Dickinson, Sydney, Australia) fitted with a 15-mW argon laser emitting light at 488 nm. The sheath fluid was Osmosol (LabAids Pty. Ltd., Sydney, Australia). The FACScalibur was equipped with forward-angle light scatter (FSC; <15o), side-angle light scatter (SSC; >15o), and three fluorescence detectors: FL1, 515 to 565 nm; FL2, 565 to 605 nm; and FL3, >605 nm. GFP expression was detected in FL1 fluorescence detectors. The cytometer was also equipped with fluorescence-activated cell sorting, which selected gated cells to be isolated. Logarithmic amplification was used throughout, and fluorescence acquisition was gated by light scatter parameters. The detection threshold was adjusted in FL1 to eliminate particles that emitted intrinsic (low) green fluorescence. Bacterial counts by flow cytometry were obtained by normalizing the numbers of events occurring in regions on dot plots that defined bacterial populations, to the volume of the sample analyzed. For cell sorting, regions were defined on bivariate dot plots that delineated distinct populations, and sort rates were set to <300 events per s. Droplets were collected onto 0.2-μm-pore-size filters (Millipore Pty. Ltd., Sydney, Australia) through a SortStage (AusFlow, Sydney, Australia) and examined by epifluorescence microscopy. Sorted samples were also diluted, and 100 μl was spread onto CCA and PSA to confirm identity and viability (see below).
Milk clearing.
Milk protein and fat were removed by using the modified milk-clearing method described by Gunasekera et al. (13). One μl of savinase (EC 3.4.21.52; Novo Nordisk Bioindustrial Pty. Ltd.) was added to 100 μl of milk, which was incubated at 30°C for 45 min. NaCl (0.15 M; 900 μl) was added, and samples were centrifuged at 10,000 × g and 22°C for 5 min. Digested proteins and the top layer containing lipids were drawn off with a micropipette, and the bacterial pellet was suspended in 100 μl of 0.15 M NaCl.
Total and viable counts based on microscopy.
The LIVE/DEAD BacLight viable fluorescent kit produced by Molecular Probes was used according to the manufacturer's instructions (Hand Book of Fluorescent Probes and Research Chemicals, 6th ed.). Membrane activity was determined by dual staining of the samples with PI (1 to 5 μl ml−1) and SYTO9 (10 μmol) and incubation of the samples for 15 min at 30°C. Stained samples were excited at 470 to 488 nm and analyzed for red and green fluorescent cells directly by using epifluorescence microscopy or by flow cytometry. A Carl Zeiss (Sydney, Australia) Axioskop 2 epifluorescence microscope fitted with ×10 eyepieces and 40× and 100× (oil immersion) objectives was used to confirm and count cells. Direct microscopic counts of bacterial suspensions were carried out by using bright-field microscopy and Thoma counting chamber procedures (8).
Statistical analyses.
Data acquired from flow cytometry were analyzed by using the Windows Multiple Document Interface flow cytometry application (WinMDI; Joseph Trotter, Salk Institute for Biological Studies, La Jolla, Calif.). Analysis of variance and Student's t test were used to detect significant differences between different methods.
RESULTS
Comparisons of DVC with a standard plate count in quantification of viable bacteria in milk after pasteurization.
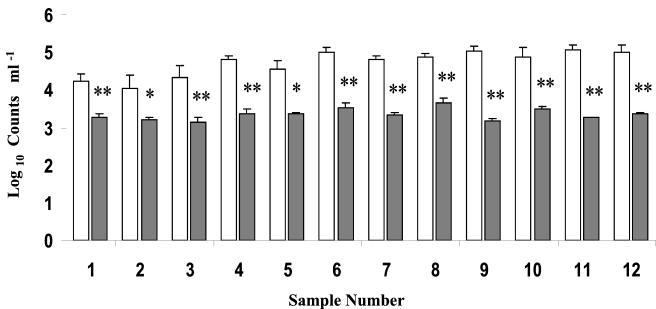
Direct viable counts (DVC) and CFU were determined in 12 separate pasteurized-milk samples obtained from a commercial HTST pasteurization unit. The number of CFU obtained was significantly (P < 0.05) lower than viable cell numbersobtained by DVC for all milk samples analyzed (Fig. 1). Overall, DVC were approximately 20-fold higher than numbers obtained by dilution plate counts.
FIG. 1.
Viability of bacteria in pasteurized milk. Viability was assayed by PI exclusion (DVC) (open bars) and CFU counts on PCA (shaded bars). Significant differences between the two methods by a two-tailed test are indicated by single asterisks (P < 0.05) and double asterisks (P < 0.01).
Analysis of GFP expression by bacteria in pasteurized milk by flow cytometry.
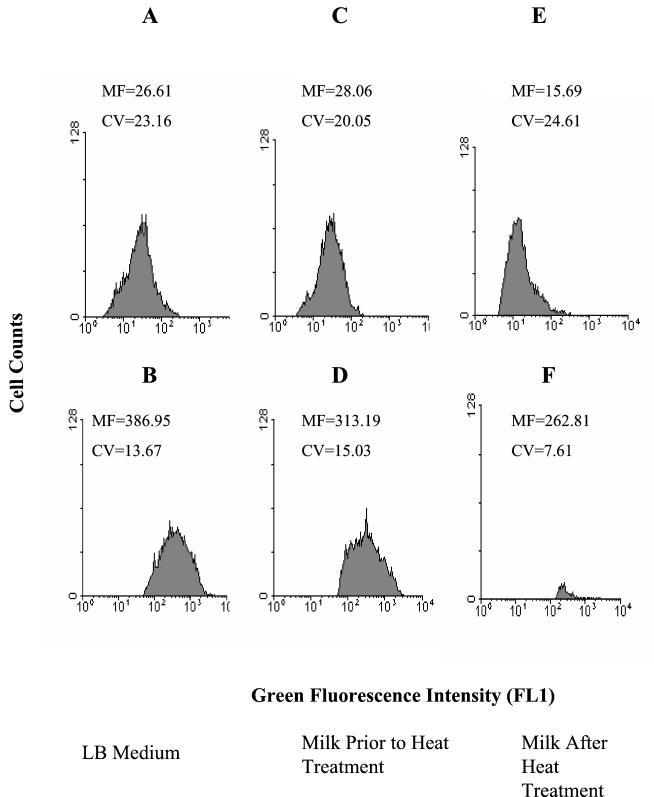
Mean fluorescence levels of GFP in P. putida cells after induction with IPTG were measured by flow cytometry. Induced cells were readily distinguished from noninduced cells because they exhibited a 10- to 15-fold-greater fluorescence intensity in the FL1 channel of the flow cytometer in both LB medium and UHT milk (Fig. 2A through D). After heat treatment, only 0.5 to 1.0% of cells were capable of expressing GFP upon induction with IPTG (Fig. 2F). These results indicate that GFP fluorescence in conjunction with flow cytometry can be used to monitor gene expression by GFP-marked bacteria in milk.
FIG. 2.
The gfp gene encoding GFP was introduced into P. putida under the control of a lac promoter and induced by IPTG. (A) Cells in LB medium before induction; (B) cells in LB medium after induction with IPTG; (C) P. putida inoculated into UHT milk; (D) P. putida inoculated into UHT milk and induced with IPTG; (E) P. putida inoculated into UHT milk, followed by heat treatment (63.5°C for 30 min); (F) P. putida inoculated into UHT milk, followed by heat treatment (63.5°C for 30 min) and induction with IPTG. MF, mean fluorescence (arbitrary scale); CV, coefficient of variation. In panel F, the dead-cell population with low green fluorescence equivalent to that seen in panel E has been gated out in order to show the high-fluorescence subpopulation distinctly.
Comparison of CFU with GFP expression by bacteria after pasteurization of milk.
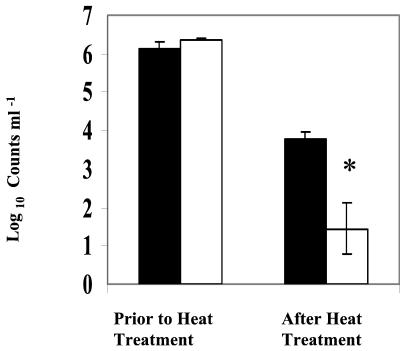
Heat treatment significantly (P < 0.01) reduced both CFU and the number of GFP-expressing E. coli cells measured by fluorescence microcopy (Fig. 3). Of the inoculated population, less than 0.005 and 2%, respectively, were capable of forming colonies and expressing GFP after heat treatment. This result suggests that of the E. coli GFP-expressing cells, only 1% was capable of forming colonies on PCA after heat treatment.
FIG. 3.
Comparison of CFU and GFP-based total counts of E. coli in pasteurized and unpasteurized milk. The asterisk indicates a significant (P < 0.05) difference. Error bars, standard errors of the means of three replicates. UHT milk was inoculated with gfp-tagged E. coli (2.5 × 106 ml−1) and heat treated (63°C for 30 min) before analysis. GFP-based viable counts were measured by epifluorescence microscopy. Solid bars, numbers of GFP-expressing cells per milliliter; open bars, numbers of CFU per milliliter.
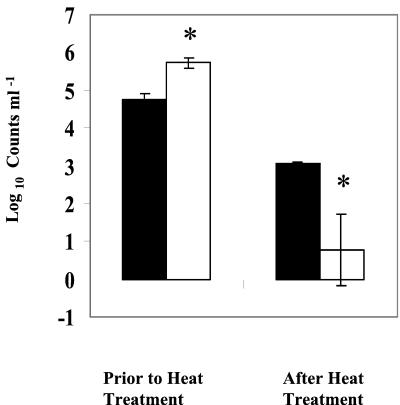
With P. putida as well, heat treatment significantly reduced both colony formation (P < 0.01) and GFP expression (Fig. 4). With an inoculum of 5.8 × 105 cells ml−1, less than 2% of the inoculated population was capable of expressing GFP after heat treatment and less than 0.02% of the inoculated population was able to form colonies (Fig. 4). This result suggests that of the P. putida GFP-expressing cells, only 6% were capable of forming colonies on PCA after heat treatment. Counterstaining of GFP-expressing E. coli or P. putida cells with PI showed that GFP-expressing bacteria excluded the dye and could be considered viable.
FIG. 4.
Comparison of CFU and GFP-based total counts of P. putida in pasteurized and unpasteurized milk. Asterisks indicate significant (P < 0.05) difference. Error bars, standard errors of the means of three replicates. UHT milk was inoculated with gfp-tagged P. putida (5.8 × 105 ml−1) and heat treated at 63.5°C for 30 min. GFP-based viable counts were measured by epifluorescence microscopy. Solid bars, numbers of GFP-expressing cells per milliliter; open bars, numbers of CFU per milliliter.
DISCUSSION
Pasteurization is widely used in the food and dairy industry both to minimize the risk from pathogens and to extend product shelf life. The objective of this research was to determine if nonculturable cells could remain biologically active following pasteurization. An inducible GFP reporter system, to measure de novo gene expression, and a fluorescent dye exclusion assay, to measure cell membrane permeability, were combined with flow cytometry and fluorescence microscopy to analyze gene expression in individual VBNC cells in real time.
E. coli and P. putida were marked with a gfp gene regulated by an inducible lac promoter together with lacIq1, which repressed the lac promoter in the absence of IPTG. Lactose in milk was unable to induce expression (Fig. 2E) due to the lack of the lacY gene (the lacY gene in E. coli was deleted), which is responsible for the synthesis of lactose permease, required for the transport of lactose across the bacterial cell membrane. IPTG does not require lactose permease for transport across the bacterial membrane and thus will induce synthesis of GFP in these systems.
In order to monitor gene expression in VBNC cells, careful controls were included to distinguish expression before and after the transition to nonculturability. Addition of nalidixic acid to cultures that were to be assayed for GFP prevented growth of the low level of culturable bacteria, so the data obtained are truly indicative of gene expression by VBNC cells. Because GFP does not interfere with the growth of cells, it is ideal for studies in which cells are stressed. In this regard, others have demonstrated the use of gfp gene fusions for monitoring gene responses of bacteria in macrophages (11, 27), the viability of bacteria in environmental samples and rhizospheres (5, 23), bacterial VBNC states (6, 20), and transfer of genetic material between bacteria in populations (7, 21) and for detection and quantification of antibiotics and mercury (15, 16). Based on these studies and the work reported here, it is clear that GFP technology in combination with flow cytometry provides a powerful means of analyzing VBNC bacterial function in complex media and environments.
In commercially available pasteurized milk, DVC, based on dye exclusion, were significantly higher (P < 0.05) than viable cell counts determined from CFU, suggesting that a significant subpopulation of cells in pasteurized milk are VBNC. To our knowledge, this work represents the first evidence for the existence of VBNC bacteria in pasteurized milk. It has been demonstrated by others that VBNC forms of medically important bacteria may conserve their pathogenicity genes and that some are capable of resuming active growth if optimal conditions are restored (4).
When strains of E. coli and P. putida, marked with gfp genes, were inoculated into UHT milk and heated at 63.5°C for 30 min, a >4-log-unit reduction in the number of CFU was observed for both organisms. By contrast, the reduction in the number of gfp-expressing cells was <2.5 log units for both organisms treated with the same conditions. These results suggest that only 1% of the E. coli cells and 6% of the P. putida cells that are capable of synthesizing GFP after heat treatment are culturable. Synthesis of introduced GFP reporter genes by E. coli and P. putida after heat treatment of milk indicates the possibility that other genes could be transcribed and translated by VBNC bacteria. In this regard, expression of proteases and lipases that are responsible for spoilage and reduced shelf life, or of toxins, may be particularly important. The results presented here also suggest that culture-based analyses significantly underestimate the fraction of metabolically active cells in pasteurized milk.
Although there are a variety of time-temperature combinations that can be used for milk pasteurization, HTST pasteurization is now a standard practice (12). The finding that a significantly high proportion of cells maintained their permeability barrier (PI exclusion) although they were unable to form colonies suggests that the biological function of bacteria can persist after the commercially relevant heat treatment process. Thus, even though the laboratory tests using the GFP-marked strains did not employ exactly the same time-temperature profile for heating as that used in the HTST process (12), we suggest that the data obtained using the laboratory model and the commercial samples support each other qualitatively.
The results demonstrate that a substantial portion of cells rendered incapable of forming colonies by heat treatment are nevertheless metabolically active and able to transcribe and translate genes de novo. This observation is important because it highlights a potential problem for milk quality and safety. Further studies, including analysis of heat-treated milk, are necessary to determine whether VBNC cells can have an effect on milk quality. For example, if VBNC psychrotrophic bacterial cells are able to recover their ability to grow in stored milk, they could produce significant spoilage enzymes (lipases and proteases).
Acknowledgments
We thank Gunnar Øregaard and Lars Hansen of the Department of General Microbiology, Institute of Molecular Biology, University of Copenhagen, for technical support and for providing GFP-marked bacteria for this study.
REFERENCES
- 1.Auty, M. A. E., G. E. Gardiner, S. J. McBrearty, E. O. O'Sullivan, D. M. Mulvihill, J. K. Collins, G. F. Fitzgerald, C. Stanton, and R. P. Ross. 2001. Direct in situ viability assessment of bacteria in probiotic dairy products using viability staining in conjunction with confocal scanning laser microscopy. Appl. Environ. Microbiol. 67:420-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barer, M. R., and C. R. Harwood. 1999. Bacterial viability and culturability. Adv. Microb. Physiol. 41:93-137. [DOI] [PubMed] [Google Scholar]
- 3.Binderova, E., and D. Rysanek. 1999. Microbial contaminants of milk processed by high-temperature short-time pasteurization. Veterinarni Medicina 44:301-307. [Google Scholar]
- 4.Bloomfield, A. F., G. S. A. B. Stewart, C. E. R. Dodd, I. R. Booth, and E. G. M. Power. 1998. The viable but non-culturable phenomenon explained? Microbiology 14:1-4. [DOI] [PubMed] [Google Scholar]
- 5.Cho, J., and S. Kim. 1999. Viable but non-culturable state of a green fluorescence protein-tagged environmental isolate of Salmonella typhi in groundwater and pond water. FEMS Microbiol. Lett. 170:257-264. [DOI] [PubMed] [Google Scholar]
- 6.Cho, J., and S. Kim. 1999. Green fluorescent protein-based direct viable count to verify a viable but non-culturable state of Salmonella typhi in environmental samples. J. Microbiol. Methods 36:227-235. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, B. B., C. Sternberg, J. B. Andersen, L. Eberl, S. Møller, M. Givskov, and S. Molin. 1998. Establishment of new genetic traits in a microbial biofilm community. Appl. Environ. Microbiol 64:2247-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruickshank, R., J. P. Duguid, B. P. Marmion, and R. H. A. Swain. 1975. The practice of medical microbiology. In Medical microbiology, 12th ed., vol. 2. Churchill Livingstone, Edinburgh, United Kingdom.
- 9.Davey, H. M., and D. B. Kell. 1996. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol. Rev. 60:641-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmasures, N., and M. Gueguen. 1997. Monitoring the microbiology of high quality milk by monthly sampling over 2 years. J. Dairy Res. 64:271-280. [DOI] [PubMed] [Google Scholar]
- 11.Dhandayuthapani, S., L. E. Via, C. A. Thomas, P. M. Horowitz, D. Deretic, and V. Deretic. 1995. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol. Microbiol. 17:901-912. [DOI] [PubMed] [Google Scholar]
- 12.Grant, I. R., H. J. Ball, S. D. Neill, and T. R. Michael. 1996. Inactivation of Mycobacterium paratuberculosis in cows' milk at pasteurization temperatures. Appl. Environ. Microbiol. 62:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunasekera, T. S., P. V. Attfield, and D. A. Veal. 2000. A flow cytometry method for rapid detection and enumeration of total bacteria in milk. Appl. Environ. Microbiol. 66:1228-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen, L. H., S. J. Sørensen, and L. B. Jensen. 1997. Chromosomal insertion of the entire Escherichia coli lactose operon into two strains of Pseudomonas, using a modified mini-Tn5 delivery system. Gene 186:167-173. [DOI] [PubMed] [Google Scholar]
- 15.Hansen, L. H., and S. J. Sørensen. 2000. Detection and quantification of tetracylines by whole cell biosensors. FEMS Microbiol. Lett. 190:273-278. [DOI] [PubMed] [Google Scholar]
- 16.Hansen, L. H., and S. J. Sørensen. 2000. Versatile biosensor vectors for detection and quantification of mercury. FEMS Microbiol. Lett. 193:123-127. [DOI] [PubMed] [Google Scholar]
- 17.International Dairy Federation. 1991. Alkaline phosphatase test as a measure of correct pasteurization. Bull. Int. Dairy Fed. 262:32-35. [Google Scholar]
- 18.Kogure, K., U. Simidu, and N. Taga. 1979. A tentative direct microscopic method for counting living marine bacteria. Can. J. Microbiol. 25:415-420. [DOI] [PubMed] [Google Scholar]
- 19.Lisle, J. T., B. H. Pyle, and G. A. McFeters. 1999. The use of multiple indices of physiological activity to access viability in chlorine disinfected Escherichia coli O157:H7. Lett. Appl. Microbiol. 29:42-47. [DOI] [PubMed] [Google Scholar]
- 20.Lowder, M., A. Unge, N. Maraha, J. K. Jansson, J. Swiggett, and J. D. Oliver. 2000. Effect of starvation and the viable-but-nonculturable state on green fluorescent protein (GFP) fluorescence in GFP-tagged Pseudomonas fluorescens A506. Appl. Environ. Microbiol. 66:3160-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Normander, B., B. B. Christensen, S. Molin, and N. Kroer. 1998. Effect of bacterial distribution and activity on conjugal gene transfer on the phylloplane of the bush bean (Phaseolus vulgaris). Appl. Environ. Microbiol. 64:1902-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver, J. D. 1993. Formation of viable non-culturable cells, p. 239-272. In S. Kjellberg (ed.), Starvation in bacteria. Plenum Press, New York, N.Y.
- 23.Ramos, C., L. Mølbak, and S. Molin. 2000. Bacterial activity in the rhizophere analyzed at the single-cell level by monitoring ribosome contents and synthesis rates. Appl. Environ. Microbiol. 66:801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengeløv, G., K. J. Kristensen, A. H. Sørensen, N. Kroer, and S. J. Sørensen. 2001. Effect of genomic location on horizontal transfer of a recombinant gene cassette between Pseudomonas strains in the rhizosphere and spermosphere of barley seedlings. Curr. Microbiol. 42:160-167. [DOI] [PubMed] [Google Scholar]
- 25.Sung, N., and M. T. Collins. 1998. Thermal tolerance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 64:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsien, R. Y. 1998. The green fluorescent protein. Annu. Rev. Biochem. 67:509-544. [DOI] [PubMed] [Google Scholar]
- 27.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 28.Veal, D. A., D. Deere, B. Ferrari, J. Piper, and P. V. Attfield. 2000. Fluorescence staining and flow cytometry for monitoring microbial cells. J. Immunol. Methods 243:191-210. [DOI] [PubMed] [Google Scholar]
- 29.Wiedmann, M., D. Weilmeier, S. S. Dineen, R. Ralyea, and K. J. Boor. 2000. Molecular and phenotypic characterization of Pseudomonas spp. isolated from milk. Appl. Environ. Microbiol. 66:2085-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]