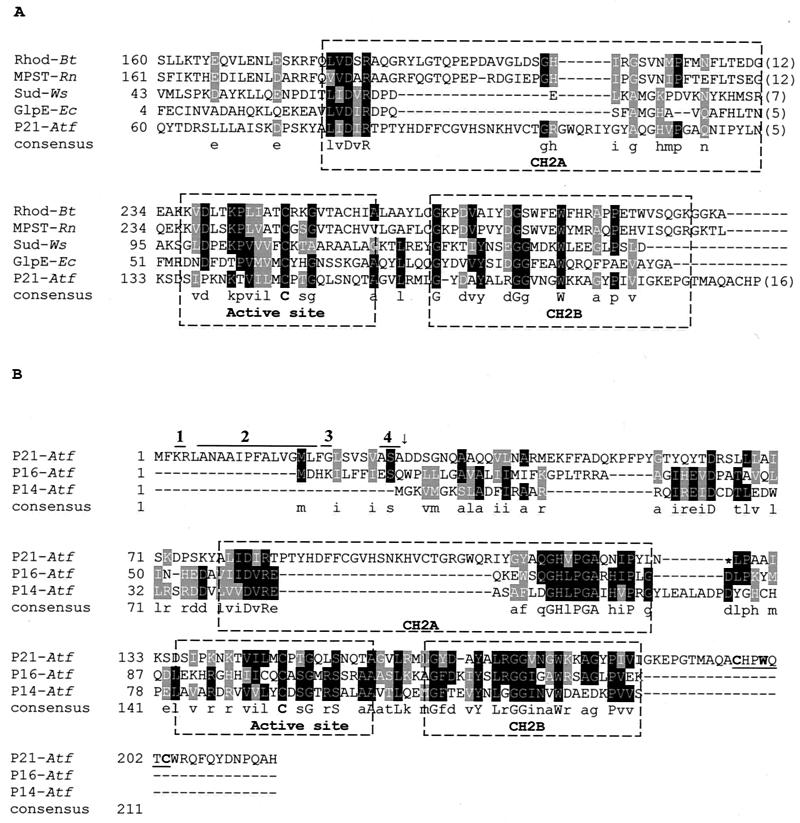
FIG. 2.
(A) Partial sequence alignment of the P21 protein of A. ferrooxidans with those of representative sulfurtransferases. The active site and two conserved structural motifs, designated CH2A and CH2B, are labeled. A consensus is indicated in which the active site presents a cysteine conserved in all of the sequences shown. Rhod-Bt, bovine liver rhodanese; MPST-Rn, rat mercaptopyruvate sulfurtransferase; Sud-Ws, sulfide dehydrogenase from W. succinogenes; GlpE-Ec, E. coli rhodanese-like protein; P21-Atf, putative periplasmic rhodanese-like protein P21 from A. ferrooxidans. (B) Sequence alignment of A. ferrooxidans proteins with similarity to rhodaneses. The P21 protein sequence contains a 28-amino-acid leader peptide. Within this leader peptide, indicated by the numbered lines above, are the following: the positively charged residues at the N terminus (line 1), a stretch of hydrophobic residues (line 2), a glycyl residue at the end of this stretch (line 3), and a consensus cleavage sequence (Ala/Gly/Ser)-X-(Ala/Gly/Ser) (line 4). The arrow indicates the site where the cleavage of the leader peptide would occur. P14-Atf, putative cytoplasmic rhodanese-like from A. ferrooxidans; P16-Atf, putative cytoplasmic rhodanase-like from A. ferrooxidans. The underlined sequence in P21-Atf indicates a putative cysteine motif known to bind iron-sulfur clusters. Accession numbers: Sud, X81642; Rhod-Bt, M58561; MPST-Rn, D50564; GlpE-Ec, M96795; P21-Atf, AJ312238; and P14-Atf and P16-Atf, derived from the genome sequence of A. ferrooxidans 23270.