Abstract
Alzheimer's disease (AD) is a progressive, chronic disease characterized by impaired cognitive function. Currently, there is no complete cure for ad; current treatments are aimed at reducing symptoms and slowing the progression of the disease. It is thought that the amount of fatty acid consumption and the balance between them may be protective against neurological diseases. In this study, it was aimed to determine the protective effects of Ω3/Ω6 polyunsaturated fatty acids ratios in Aβ1–42-induced ad model in human neuroblastoma (SH-SY5Y) cells. The viability of cells was determined by MTT assay. The percentage of apoptotic cells was determined by FITC-conjugated Annexin-V/PI. ROS, MMP and cell cycle analysis were performed by flow cytometry. The amount of acetylcholinesterase enzyme was measured with a commercial kit. 48 h after the application, a statistically significant decrease was observed in the MTT test in the 1/1, 1/2 and 1/8 groups and in the amount of AChE in the 1/4, 1/8 and 1/16 groups. According to apoptosis findings, all ratios were observed to reduce cell viability compared to the control group. ROS and MMP levels were detected to decrease in all groups compared to the control group. The highest neuroprotective effect against oxidative stress was observed at 1/1 dose. Aβ1–42 induced blockade of the cell cycle was observed to be partially corrected by 1/8 dose. As a result, it can be said that Ω6 and Ω3 fatty acids, when used in 1/4 and 1/8 doses can provide a protective effect against Alzheimer's disease.
Keywords: Alzheimer, apoptosis, MMP, cell cycle, Ω3/ Ω6, ROS
Introduction
Alzheimer's disease (ad) is a progressive, chronic disease characterized clinically by deterioration in cognitive functions, including memory and logical thinking processes.1 According to the World Health Organization (WHO, WHO) 2022 data, there are approximately 55 million patients with dementia in the world, and 60–70% of these patients have ad. These data show that the number of patients with ad is expected to increase to 78 million in 2030 and 139 million in 2050.2
The pathogenesis of ad is characterized by the accumulation of Amyloid beta (Aβ) protein, amyloid/senile plaques, and neurofibrillary tangles (NFTs) due to the accumulation of hyperphosphorylated Tau protein. Accumulation of Aβ initiates a series of mechanisms that cause damage such as oxidative stress, cell membrane disruption, disturbances in mitochondrial metabolism, cell cycle defects, abnormal protein folding and DNA damage, resulting in degeneration and neuronal death in synapses.3–5
Dietary factors play a key role in metabolism and affect neuroprotective cytokine release. Changes in diet composition and circulating fatty acid levels play a role in hypothalamic inflammation. The amount of fatty acids consumed and the balance between them may protect against neurological diseases. Approximately 60% of the mammalian brain consists of phospholipids.6 25% of the fatty acids in the brain consist of the omega 3 (Ω3) fatty acid docosahexaenoic acid (DHA) and the omega 6 (Ω6) fatty acid arachidonic acid (AA). Dietary alphalinolenic acid (ALA) consumption is important for the synthesis of these long-chain fatty acids in the body.7 Long-chain PUFA levels in the brain have been highly correlated with dietary PUFA and long-chain PUFA intake. EPA, DHA and AA are also precursors of many bioactive lipids known as docosanoids and eicosanoids, which are active regulators in the inflammatory mechanism.
The balance of Ω6 and Ω3 fatty acids is very important for homeostasis and healthy development throughout the life cycle. Changes in the ratio between Ω6/Ω3 fatty acids in the diet cause different metabolic effects. The balance between fatty acids and the metabolic effects of this balance are the subject of various studies. Different diets contain different fatty acid ratios, the ratio Ω6/Ω3 = 15–20/1 is called the “Western-style diet.”8 A high ratio of Ω6/Ω3 is associated with the risk of cardiovascular disease, obesity, diabetes, cancer and inflammatory autoimmune diseases. While the ratio Ω6/Ω3 = 13/1 is associated with the presence of chronic disease, it is reported that reducing this ratio to 7/1 protects against cancer and cardiovascular diseases. In addition, this ratio of 7/1 is named as one of the healthy nutrition goals.
Currently, there is no curative treatment for any type of neurodegenerative disease and despite many promising innovations in this field, treatment strategies fail to prevent neuronal loss. Disease-modifying treatments focus on relieving symptoms and slowing the progression of the disease. There is accumulating scientific evidence on the possible effectiveness of PUFA supplementation in neurodegenerative disorders. Although dietary recommendations are far from being accepted as a treatment for neurodegenerative disorders, they can alleviate some symptoms and, most importantly, slow down the cognitive and physical decline that has the greatest impact on quality of life.9 Numerous studies have highlighted the anti-inflammatory effects of PUFAs and their potential use as therapeutic agents in neurodegenerative diseases such as Parkinson's disease (PD), Dementia, ad, Multiple Sclerosis (MS), Huntington's disease and Amyotrophic Lateral Sclerosis (ALS).10 It is thought that they may have positive contributions to the course of neurodegenerative diseases such as ad and that changing the diet of individuals, considering genetic factors, may have an effect on slowing down the progression of the disease. In our study, we aimed to investigate the protective effects of different Ω3/Ω6 fatty acid ratios, created using EPA from Ω3 fatty acids and LA from Ω6 fatty acids, on cell viability, intracellular ROS amount, apoptosis rates and reduced mitochondrial membrane potential in the in vitro ad model created with Aβ1–42 and SH-SY5Y cells.
Materials and methods
Cell culture
The SH-SY5Y (CRL-2266™) cell line was used in this work. Cells were cultured at 37 °C in 5% CO in DMEM (Capricorn Scientific, Germany) with 10% FBS (Capricorn Scientific, FBS-HI11B, Germany), 100 IU/mL penicillin (Capricorn Scientific, PS-B, Germany), 100 μg/mL streptomycin (Capricorn Scientific, PS-B, Germany), and passaged at 70–80% confluence.
The medium containing the cells that reached 80–90% filling level was removed by aspirating with a pipette. 3–5 mL of 0.05% trypsin was added to the flasks and kept in a 37oC 5% CO2 incubator for 2–3 min to separate them from the ground. The effect of trypsin was stopped by adding FBS to the cells that separated from the surface and the cells collected in a 15 mL centrifuge tube were centrifuged at 1000 rpm for 5 min to remove the supernatant. The cells resuspended with 6–7 mL medium were transferred to new culture dishes and kept in a 37oC 5% CO2 incubator. The cells were monitored with an inverted microscope and the medium was changed with fresh medium every 3 days. When the 70–80% filling level was reached, the passaging process was repeated.
Determination of cell number and measurement of viability
In each stage of our study, live cells were counted to determine the amount of cells to be used. Trypan blue was used for cell counting. While the cytoplasm of dead cells was stained blue because their membrane integrity was disrupted, live cells were not stained because they did not take the dye into the cell. 10 μL of trypan blue and 10 μL of cell suspension were mixed and added to both compartments of the Thoma slide as 10 μL. Using an inverted microscope, cells in 4 compartments of the Thoma slide consisting of 16 equal squares were counted and the average was taken.
The number of viable cells was calculated using the following formula:
Number of live cells per millilitre = [Average cell count × (104) × Dilution ratio]/9.
Preparation of amyloid β1–42
500 μg Amyloid β1–42 (Item no:20574, Cayman) was diluted with 27.7 ml DMSO according to the kit protocol to obtain a 4 mM stock solution. The obtained stock solution was applied to the cells for 24, 48 and 72 h in doses of 2.5 μM, 5 μM, 10 μM, 50 μM, 100 μM, 200 μM, 400 μM by serial dilution in DMEM medium.
The half-maximum inhibitory concentration (IC50) of Amyloid β1–42 was calculated as 100 μM.
MTT assay
Cells were seeded in a 96-well plate at 104 cells per well and incubated for 24 h at 37 °C and 5% CO2. Cells were treated with a combination of ω-6 and ω-3 fatty acids. After 48 h of incubation, each well received 20 μL of MTT solution and incubated for 3–4 h. After incubation, the supernatant was gently removed. Each well was treated with 100 μL of isopropyl alcohol and incubated for 2 h at room temperature in the dark. Then the plate was shaken and read at 570 nm. MTT testing was performed for both AB1–42 dose determination and subsequently for all PUFA applications.
Acetylcholinesterase (AChE) quantification
Determination of AChE amount (Elabscience Biotechnology Inc. Texas, USA) is based on sandwich ELISA method. Wells are coated with AChE antibodies. AChE in samples binds to antibodies. Streptovidin-HRP is added for immune complex formation and antigen–antibody complex is attached to wells. Absorbance of colored complex formed by chromogen addition is measured at 450 nm.
Preparation of fatty acids and experimental groups
Eicosapentaenoic acid (EPA) as the Ω-3 fatty acid and linoleic acid (LA) as the Ω-6 fatty acid were used in this study. Six experimental groups were created. The first group was the control group that was not subjected to treatment. The Ω-3: Ω-6 ratios were 1/1, 1/2, 1/4, 1/8 and 1/16, respectively.
200 μl of EPA (Item no:90110, Cayman) was diluted with DMEM according to the kit protocol to obtain a 4 mM stock solution. The stock solution we obtained was applied to the cells for 48 and 72 h in doses of 5 μM, 10 μM, 25 μM, 40 μM, 50 μM by serial dilution in DMEM medium.
The existing stock solution of LA (PC1003367601, Sigma) which is 3.241 M was diluted with DMEM according to the kit protocol to obtain a 4 mM stock solution. The stock solution we obtained was applied to the cells at doses of 5 μM, 10 μM, 25 μM, 40 μM, 50 μM by serial dilution in DMEM medium for 48 and 72 h.
Determination of fatty acid ratios
After the application of Ω3 and Ω6 fatty acids, the working dose was determined as 50 μM by looking at the MTT and Annexin V data. The Ω3/Ω6 ratios were determined as 1/1, 1/2, 1/4, 1/8 and 1/16 and the working doses were added to the culture media after diluting the stock solutions with DMEM medium.
Annexin-V analysis
Measurement of Annexin V bound to the cell surface as an indicator of apoptosis should be performed in conjunction with a staining test to determine the integrity of the cell membrane.It distinguishes whole cells (FITC-/PI-), apoptotic cells (FITC+/PI-), and necrotic cells (FITC+/PI+). Annexin V analysis was performed using Annexin V Detection Kits (BD Pharmingen™ FITC and PE Annexin V Apoptosis Detection Kits Cat. Nos. 556,570) To analyze apoptosis, cells (1 × 105) were trypsinized after being treated with varying ratios of ω-6 and ω-3 fatty acids after 48 h. The cell pellet was then dissolved in 100 μL binding solution and added to 5×105 cells in 500 mL. Then, 5 μL of Annexin and 20 μL of PI were added and pipetted. After 30 min of incubation in the dark at room temperature, 400 μL of 1X binding buffer was added and examined in BD Accuri C6 (BD Biosciences) flow cytometer.
Analysis of ROS levels
Determination of intracellular reactive oxygen species was performed using ROS Detection Kit (THORVACS Biotechnology, Cat#; ROS-100 T). Cells prepared in the experimental groups created in the SH-SY5Y cell line were collected by trypsinization method.1x105 cells were suspended in PBS, centrifuged at 1000 rpm for 5 min, and the supernatant was discarded. Cell pellets were resuspended in 200 μL of 100 mM DCF-DA reagent and incubated at 37 °C for 30 min in the dark. The cells were then centrifuged at 1000 rpm for 5 min and the supernatant was discarded. The pellet was resuspended in 100 μL PBS and analyzed using the FL1 channel on the BD Accuri C6 (BD Biosciences) flow cytometry device.
Cell cycle analysis
Cell cycle analysis was performed using the Cell Cycle Kit (THORVACS Biotechnology, Cat#; CCK-100 T). The cells prepared in the experimental groups created in the SH-SY5Y cell line were prepared by centrifuging twice with cold PBS at 500 × g for 5 min to reach 1×106 cells/ml. The cell pellet was fixed in 200 μL of 70% ethanol at 4 °C for 30 min on ice and then washed twice with PBS at 800 × g for 5 min. 200 μL staining solution was added to the cells incubated with 50 μL enzyme solution at 37 °C for 30 min and the cells were kept at 37 °C for another 15–30 min. The stained cells were analyzed using the FL3 channel on the BD Accuri C6 (BD Biosciences) flow cytometry device.
MMP measurement
Mitochondria are involved in many processes such as cell signaling, cell cycle control, cellular growth, cell differentiation, metabolite transport, and cell death. Disruption of mitochondrial transmembrane potential is one of the earliest intracellular events that occur in the induction of apoptosis. Analysis of mitochondrial membrane potential was performed using the MMP Kit (THORVACS Biotechnology, Cat#; MMPAK-100 T). Cells prepared in the experimental groups created in the SH-SY5Y cell line were collected by trypsinization. The cells, which were washed twice with cold PBS by centrifuging at 500 × g for 5 min, were prepared to be 1×106 cells/ml. They were labeled with 10 μL JC-1 dye and incubated at 37 °C for 30 min and analyzed on the BD Accuri C6 (BD Biosciences) flow cytometry device.
Statistical analysis
Mean and standard deviation (mean ± sd) were used for continuous data as descriptive statistics. The suitability of continuous data for normal distribution was checked with the Kolmogorov–Smirnov test. The distributions of variables in two groups that were suitable for normal distribution were compared with the Student's t test, and those that were not suitable for normal distribution were compared with the Mann–Whitney U test. The distributions of variables that do not comply with normal distribution in three or more groups were analyzed with the Kruskal-Wallis test, and the formula developed by Conover (1980) was used as the multiple comparison test. Data were analyzed with the IBM SPSS 23 (IBM SPSS Inc, Chicago, IL) package program. The significance level was accepted as P < 0.05.
Results
Effect of amyloid β1–42 on SH-SY5Y cell line
MTT test
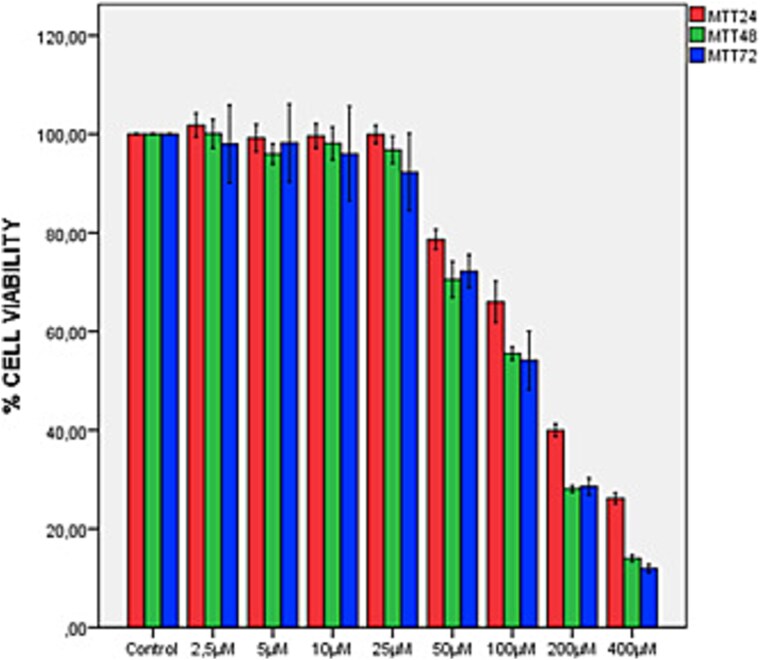
The difference between the mean MTT measurements of the 8 groups at the 24th, 48th and 72nd hours was found to be statistically significant (F(7,40) = 970.179, P < 0.001; F(7,40) = 1288.580, P < 0.001; F(7,40) = 185.910, P < 0.001, respectively) (Fig. 1). After using the multiple comparison test Bonferroni correction, when the control group was accepted as 100%, the percentage decreases in the 50 μM, 100 μM, 200 μM and 400 μM groups were found to be statistically significantly different in each application (P < 0.05). According to the study data, 48 and 72 h applications of Aβ1–42 were found to be similar. The effective concentration of Aβ1–42 that can create an ad model on SH-SY5Y cells but does not have a high cytotoxic effect was determined as 100 μM.
Fig. 1.
MTT test results of 24–48-72 h effect of Aβ1–42 on SH-SY5Y neuroblastoma cell line.
Inverted microscope image
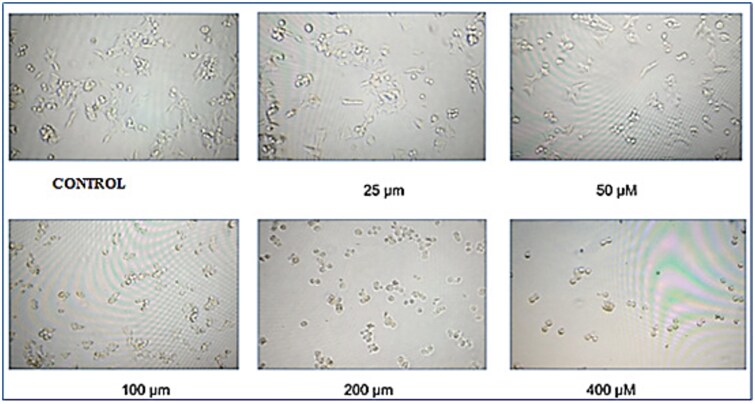
After 48 h of Amyloid β1–42 exposure, images of SH-SY5Y cells were taken with an inverted microscope (Nikon F12, Japan) (Fig. 2). A decrease in the number of SH-SY5Y cells was observed in line with the increase in the Amyloid β1–42 dose. Quantitative results obtained with MTT test were confirmed visually.
Fig. 2.
Inverted microscope image of the groups treated and not treated with Aβ1–42 on SH-SY5Y cell lines at the end of 48 h.
Protein levels of AChE
As a result of the application of 100 μM Aβ1–42 to SH-SY5Y cells, it was observed that AChE protein levels was induced and increased by 62% compared to the control group without Aβ1–42 application (χ2 = 18.657, P < 0.005) (Fig. 3).
Fig. 3.
AChE protein levels in the in vitro ad model established in SH-SY5Y cells after Aβ1–42 application.
Effect of various doses of PUFA (Ω3 and Ω6) applications for 48 hours
MTT test
When the difference between the means of the groups with different Ω3 concentrations and the control group without Ω3 was examined at the end of the 48th hour, a statistically significant difference was found between the groups (χ2 = 50.110, P < 0.001). Conover formula was used to find the source of the difference. When the control group was accepted as 100%, a statistically significant difference was found in all groups, except the Ω3–50 μM and Ω3–10 μM groups, compared to the control group (P < 0.001) (Fig. 4a).
Fig. 4.
MTT test results after application of various doses of Ω3 a) and Ω6 b) on SH-SY5Y cell line for 48 h.
When the difference between the means of the groups with different Ω6 concentrations and the control group without Ω6 was examined at the end of the 48th hour, a statistically significant difference was found between the groups (χ2 = 50.110, P < 0.001). When the control group was accepted as 100%, a statistically significant difference was found in all groups except the Ω6–50 μM and Ω6–10 μM groups compared to the control group (P < 0.001) (Fig. 4b).
Apoptosis levels
For the Annexin V test, the survival value was determined as 98.2% in the control group (Aβ–control) that did not receive Aβ1–42 for 48 h, while the survival value was determined as 97% in the control group (Aβ + control) that received Aβ1–42 for 48 h. As a result of Aβ1–42 application for 24 h and then Ω3 and Ω6 application at various doses for 48 h, apoptosis values were determined as 97.9%, 93.9%, 95%, 81.7%, 97.1% for Ω3 and 94.8%, 95.8%, 96.2%, 93.5%, 98.2% for Ω6 at 5 μM, 10 μM, 25 μM, 40 μM and 50 μM, respectively (Fig. 5).
Fig. 5.
Annexin V images of the effects of 48-h applications of various doses of PUFA (Ω3 and Ω6) on cell viability in vitro ad model created in SH-SY5Y cells using Aβ1–42.
Effect of various ratios of PUFA (Ω3/Ω6) applications for 48 h
MTT test
According to both the results of the MTT test and the Annexin V analysis of PUFA 48-h applications, Ω3 and Ω6 doses were selected as 50 μM to be used in the following experiments.
At the end of the 48th hour, the difference between the means of Ω3/Ω6 ratios compared to the control group was analyzed and a statistically significant difference was found between the groups (χ2 = 27.450, P < 0.001). Conover formula was used to find the source of the difference. When the control group is accepted as 100%, a statistically significant decrease is observed in the 1\1, 1\2 and 1\8 experimental groups, and an increase is observed in the 1\4 and 1\16 groups (P < 0.001) (Fig. 6).
Fig. 6.
MTT test results of the effect of 48-h application of PUFA at various rates on the SH-SY5Y cell line.
Inverted microscope images
Images of the cells were taken with an inverted microscope ((Nikon F12, Japan) after 24-h Aβ1–42 and 48-h various Ω3/Ω6 ratios application (Fig. 7). The images obtained were compared with the control groups and evaluated visually. According to the Aβ + control image, it was observed that the number of cells was the highest in the 1\1 group and the lowest in the 1\16 group.
Fig. 7.
Inverted microscope image of the effect of 48-h applications of PUFA (Ω3/Ω6) ratios on SH-SY5Y cell line.
Protein levels of AChE
When compared to the control group, AChE protein levels of cells applied with different Ω3/Ω6 ratios was found to be statistically significantly lower (χ2 = 16.596, P < 0.005). When compared to the control group, a statistically significant decrease was observed in the 1/4, 1/8 and 1/16 experimental groups (P < 0.01, P < 0.001 and p < 0.005, respectively). A statistically significant decrease was observed in the 1/4 group compared to the 1/1 group (P < 0.05) and in the 1/8 group compared to the 1/1 and 1/2 groups (P < 0.01 and P < 0.05, respectively) (Fig. 8).
Fig. 8.
Effect of PUFA (Ω3/Ω6) ratios on AChE protein levels. aP < 0,01 compared with the control group. bP < 0,001 compared with the control group. cP < 0,005 compared with the control group. dP < 0,05 compared with the 1/1 group. eP < 0,01 compared with the 1/1 group. fP < 0,05 compared with the ½ group.
Apoptosis levels
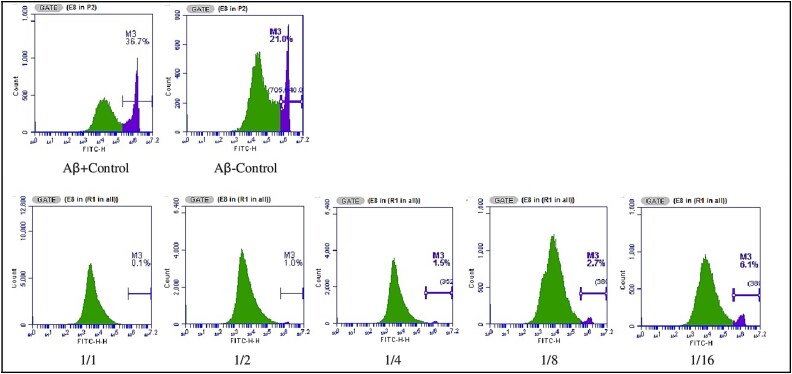
For the annexin V test, the survival value in the Aβ-control group was determined as 93.7%, while the survival value in the Aβ + control group was determined as 95.7%. As a result of the application of various Ω3/Ω6 ratios, apoptosis values in the 1/1, 1/2, 1/4, 1/8 and 1/16 groups were determined as 95.6, 81.4, 86.2, 32.2 and 19.8%, respectively (Fig. 9).
Fig. 9.
AnnexinV analysis images of the effect of PUFA (Ω3/Ω6) ratios on cell viability in vitro ad model created in SH-SY5Y cells using Aβ1–42.
The level of intracellular ROS
According to the ROS analysis results, while the ROS level in the Aβ-control group was 21, the ROS level in the Aβ + control group increased by 1.74 times to 36.7. As a result of the application of various Ω3/Ω6 ratios, it was observed that the ROS levels in the 1/1, 1/2, 1/4, 1/8 and 1/16 groups decreased significantly compared to the Aβ + control group (Fig. 10).
Fig. 10.
ROS level analysis images of cells in vitro ad model created in SH-SY5Y cells using Aβ1–42 after 48 h of PUFA (Ω3/Ω6) ratio applications.
MMP analysis
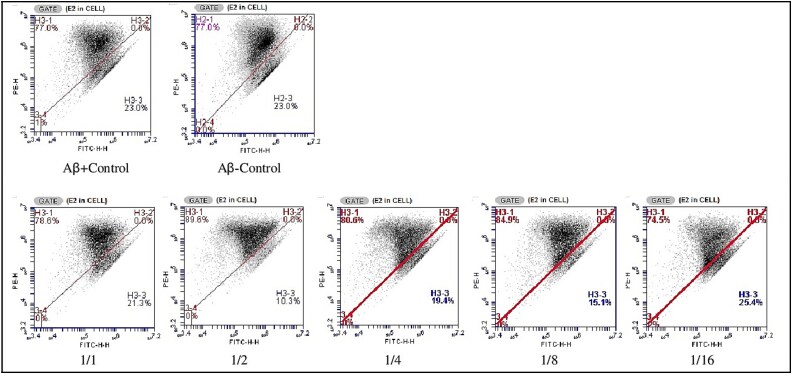
According to the MMP analysis results, the rate of MMP affected cells in both the Aβ-control group and the Aβ + control group was found to be 23%. As a result of the application of various Ω3/Ω6 ratios for 24 h, the percentage of MMP affected cells compared to the Aβ + control group was calculated as 21.3, 10.3, 19.4, 15.1 and 25.4% for the 1/1, 1/2, 1/4, 1/8 and 1/16 groups, respectively (Fig. 11).
Fig. 11.
MMP analysis images of cells in vitro ad model created in SH-SY5Y cells using Aβ1–42 after 48 h of PUFA (Ω3/Ω6) ratio applications.
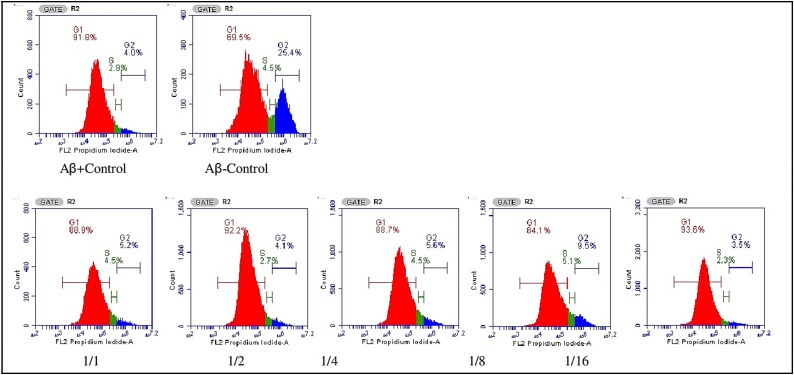
Cell cycle analysis
According to the cell cycle analysis images, the 1st peak represents G1, the 2nd peak represents G2, and the section between G1 and G2 represents the S phase. Cell cycle arrest is a critical tool used by the cell to allow DNA damage to be repaired before the cell continues its division cycle. In our study, the G1 phase rate was calculated as 69.5% in the control group without Aβ1–42 application, while this rate increased to 92% in the control group with Aβ1–42 application. For this reason, the cell rates in the S and G2 phases decreased from 4.5% to 2.9% and from 25.4% to 4.0%, respectively. These results are the result of Aβ1–42 application, which causes the cell cycle to stop at the G1-S transition. It was observed that the application of Ω3/Ω6 ratios caused an increase in the S and G2 phases in the 1/8 experimental group. The S phase increased from 2.9% to 5.1%, and the G2 phase increased more than 2-fold to 9.5%. No significant increase was observed in the other experimental groups. These results show that the cell cycle arrest caused by Aβ1–42 application can be partially corrected by using the Ω3/Ω6 = 1/8 ratio (Fig. 12).
Fig. 12.
Cell cycle analysis images of cells in vitro ad model created in SH-SY5Y cells using Aβ1–42 after 48 h of PUFA (Ω3/Ω6) ratio applications.
Discussion
Aβ1–42-induced cytotoxicity is a valid in vitro neurodegeneration model for ad.11 The accumulation of Aβ causes microglia activation, astrocyte recruitment, and continuous production of proinflammatory cytokines. These cytokines promote Aβ synthesis and amyloid formation, triggering a cycle of inflammation and amyloidogenesis.12After PUFA is applied to the in vitro ad model created in SH-SY5Y cells, cell viability responds dose-dependent. Studies indicate that Aβ1–42 demonstrates cytotoxic effects when administered to SH-SY5Y cells at concentrations of 2 μM and 0–50 μM for 48 h.13 In our study, we determined that a concentration of 100 μM Aβ1–42 can effectively create an in vitro ad model in human SH-SY5Y cells without causing significant cytotoxicity.
Most of the current drugs used clinically for the treatment of ad target the AChE enzyme, and one of the therapeutic targets in ad is the cholinergic system.14 In our study, Aβ1–42 application increased the amount of AChE protein compared to the control group. On the other hand, all PUFA applications were found to have inhibitory effects. Statistically significant decreases were observed in the 1/4, 1/8 and 1/16 experimental groups compared to the control group.
For PUFA applications in the in vitro ad model, the MTT test and Apoptosis analysis results were evaluated together after EPA doses of 5, 10, 25, 40 and 50 μM and LA doses of 5, 10, 25, 40 and 50 μM were applied for 48 h. Since the MTT results were close to each other, the decision was made based on the apoptosis results, and the doses to be used for both Ω3 and Ω6 were determined as 50 μM. In a study conducted with a different human neuroblastoma cell line, the antiproliferative dose of Ω3 EPA was found to be 35 μM.15 In studies conducted with human SH-SY5Y cell line, DHA was used at a dose of 12.5 μM as Ω3.16 while another study used EPA and ALA at a dose of 30 μM.17 In another in vitro study, the dose of EPA application to the SH-SY5Y cell line was selected as 50 μM.18 Unlike the last one, our study is an in vitro ad model created with Aβ1–42 in SH-SY5Y cells, and no study using PUFA applications and Ω3 and Ω6 ratios in this model was found.
In various animal studies examining the effects of ad and fatty acids, it has been shown that dietary supplementation with Ω3 fatty acids DHA and EPA improves the cognitive functions of animals.19 Human studies have also shown promising improvements in the cognitive functions of ad individuals taking Ω3.20 Unlike the benefits of Ω3 fatty acids, some studies conducted with Ω6 fatty acids LA mention their negative effects.21 Consumption of high LA oils has been reported to contribute to low-grade inflammation, oxidative stress, endothelial dysfunction, and atherosclerosis.22 In an in vitro study to determine the effect of Ω6 LA and AA and Ω9 fatty acid oleic acid (OA) on ad pathology, it was shown that all three fatty acids induce polymerization of both tau and Aβ.21 Many Ω6-derived prostaglandins and leukotrienes have been reported to increase cellular proliferation, promote angiogenesis, inhibit apoptosis, and stimulate inflammation that suppresses immune responses.23 It has been reported that the Ω3/Ω6 ratio in the brain is targeted at 1/1–1/2; that the dietary Ω3/Ω6 ratio is between 1/2–1/3, which helps prevent inflammation and should not exceed 1/5; and that ratios of 1/10 and above have detrimental effects on human health.24 As a result of applying Ω3/Ω6 ratios for 48 h, varying results were observed in cell viability based on the MTT test outcomes. When the control group is accepted as 100%, a statistically significant decrease is observed in the 1\1, 1\2 and 1\8 experimental groups, and an increase is observed in the 1\4 and 1\16 groups. In the apoptosis results, it was observed that after 48 h of applying Ω3/Ω6 ratios, the percentage viability decreased as the dose of Ω6 increased. The highest percentage of viability was observed at 1/1.
Studies have reported that Aβ increases free radical production in neuronal cells, reduces antioxidant enzyme activities, and leads to oxidative stress and cell death.25 Membrane lipids are more prone to PUFA peroxidation, thus increasing the permeability of the cell membrane to promote apoptosis in the cell. A study showed that Ppt1-KO neurons treated with a combination of Ω3 and Ω6 had significantly reduced ROS levels compared to the untreated group.26 A different study showed that 24-h applications of LA and EPA increased ROS levels in embryonic stem cells.27 In a study, it was stated that Ω6 fatty acids cause cell death by inducing ROS production and mitochondrial damage.28 In our study, consistent with the literature, the highest ROS levels were observed in the control group where Aβ1–42 was applied. As a result of the application of PUFA at different Ω3/Ω6 ratios for 48 h, it was determined that ROS levels in all groups decreased compared to the control group. In line with the literature, our study demonstrated that the 1/1 ratio application exhibited the most significant neuroprotective effect against Aβ1–42-induced cell death and oxidative stress.29 PUFA reduced ROS levels by activating intracellular antioxidant enzyme systems and inhibiting lipid peroxidation.
PUFAs modify the fatty acid side chains of cardiolipin and consequently cause remodeling of mitochondrial phospholipids.28 A study with EPA and DHA reported positive effects of feeding krill oil with high concentrations of phospholipids on mitochondrial membrane potential.30 Studies have shown that as Ω6 intake increases proportionally, mitochondrial functions deteriorate, basal and maximum respiration decrease, and mitochondrial activity, including proton leak and ATP production, decreases.31 Mitochondrial membranes have high DHA content. An animal study reported that EPA regulates ROS and apoptosis by increasing mitochondrial phospholipid unsaturation.32 In an in vivo study, it was shown that Ω3 PUFA added to animal diets reduced cardiolipin levels and cell apoptosis.20 Studies show that DHA is crucial for ATP synthesis via oxidative phosphorylation.33 It has been demonstrated that Ω3 fatty acids increase manganese-dependent superoxide dismutase activity while decreasing mitochondrial oxidative stress and cytochrome c oxidase activity.34 In our study, results consistent with the literature were found. MMP measured after PUFA application to the cell culture in the in vitro ad model created with Aβ1–42 in human SH-SY5Y cells responds dose-dependently. The MMP level was found to be highest in the control group, similar to the results observed for ROS and apoptosis. The highest neuroprotective effect against cell death and mitochondrial membrane damage was found in the Ω3/Ω6 = 1/2 experimental group. In particular, it has been observed that Ω3 PUFA can change membrane permeability and regulate mitochondrial membrane function by affecting membrane potential.
Determining the number of cells in certain stages of the cell cycle (G0/G1, S and G2/M) in a cell population is very important for screening compounds that affect cell growth and division.
In our study, it was observed that Aβ1–42 application stopped the cell cycle at the G1-S transition. In addition, in our study, it was observed that the application of Ω3/Ω6 ratios caused an increase in S and G2 phases in the 1/8 experimental group. In addition, the S phase increased from 2.9 to 5.1%, and the G2 phase increased more than 2-fold to 9.5%, while no significant increase was observed in the other experimental groups. Furthermore, the S phase increased from 2.9 to 5.1%, and the G2 phase increased more than 2-fold to 9.5%, while no significant increase was observed in the other experimental groups. These results show that the cell cycle arrest caused by Aβ1–42 application can be partially corrected by using the Ω3/Ω6 = 1/8 ratio.
Conclusion
Alzheimer's disease (ad) is a progressive, chronic disease characterized clinically by deterioration in cognitive functions, including memory and logical thinking processes. Currently, there are no cures for any type of neurodegenerative disease, and despite numerous promising advances in the field, treatment options fail to halt neuronal death.
Disease-modifying therapies aim to reduce symptoms and slow disease progression. It is thought that dietary composition and circulating fatty acid levels, consumption amounts and the balance between them may be protective against neurological diseases. There is a lot of scientific evidence about the possible effectiveness of PUFA supplementation in neurodegenerative disorders. According to our study results, we think that the use of Ω3 and Ω6 at 1/1 and 1/4 doses, in line with the literature, may be useful in protecting against neurodegenerative diseases such as ad and may slow down the cognitive and physical decline that has the greatest impact on the quality of life of patients.
Contributor Information
Mualla Pınar Elci, University of Health Sciences, Gulhane Institute of Health Science, Stem Cell Laboratory, 06010, Ankara, Türkiye.
Sema Ören, University of Health Sciences, Gulhane Institute of Health Science, Molecular Research Laboratory, 06010, Ankara, Türkiye.
Ece Miser-Salihoglu, Department of Biochemistry, Gazi University, Faculty of Pharmacy, Etiler, 06330, Ankara, Türkiye.
Sevgi Yardim-Akaydin, Department of Biochemistry, Gazi University, Faculty of Pharmacy, Etiler, 06330, Ankara, Türkiye.
Author contributions
M.P.E. and S.Y.A conceived and planned the experiments, M.P.E., and S.Ö. carried out the experiment, M.P.E. and S.Y.A data analysis, M.P.E., S.Y.A and E.M.S wrote the main manuscript; S.Y.A revised the manuscript. All the authors have read and approved the final manuscript.
Funding
This study was supported by the Scientific Research Project Foundation of Gazi University, Türkiye. (Project No.: TDK-2023, Project ID: 8380.
Availability of data and material
The data analyzed during this study are available from the corresponding author upon reasonable request.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Erkekoğlu P, Baydar T. Güncel in vitro sitotoksisite testleri. HUJPHARM. 2021:41:45–63. [Google Scholar]
- 2. Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q. Alzheimer’s disease: epidemiology and clinical progression. Neurol Ther. 2022:11:553–569. 10.1007/s40120-022-00338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xia W, Zhang J, Perez R, Koo EH, Selkoe DJ. Interaction between amyloid precursor protein and presenilins in mammalian cells: implications for the pathogenesis of Alzheimer disease. PNAS. 1997:94:8208–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuszczyk M. Cell type specific uptake and clearance of wita-amyloid peptides. Acta Neurobiol Exp. 2009:3:341. [Google Scholar]
- 5. Hampel H et al. Biological markers of amyloid β-related mechanisms in Alzheimer's disease. Exp Neurol. 2010:223:334–346. 10.1016/j.expneurol.2009.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. JLR. 1968:9:570–579. [PubMed] [Google Scholar]
- 7. Sambra V, Echeverria F, Valenzuela A, Chouinard-Watkins R, Valenzuela R. Docosahexaenoic and arachidonic acids as neuroprotective nutrients throughout the life cycle. Nutrients. 2021:13:986. 10.3390/nu13030986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol. 2011:44:203–215. 10.1007/s12035-010-8162-0 [DOI] [PubMed] [Google Scholar]
- 9. Avallone R, Vitale G, Bertolotti M. Omega-3 fatty acids and neurodegenerative diseases: new evidence in clinical trials. Int J Mol Sci. 2019:20:4256. 10.3390/ijms20174256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calviello G et al. Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases. Biomed Res Int. 2013:2013:743171. 10.1155/2013/743171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gürsoy Çalan Ö, Akan P, Alper Bağrıyanık H, Fadıloğlu M. (). Amiloid Beta Peptit ve Nöroaktif steroid Uygulamasına Karşı İnsan ve Sıçan Nöronal Hücrelerinin vital Yanıtlarının Değerlendirilmesi. J Neurol Sci. 2014:31:1. [Google Scholar]
- 12. Akiyama H et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000:21:383–421. 10.1016/S0197-4580(00)00124-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei W, Wang X, Kusiak JW. Signaling events in amyloid β-peptide-induced neuronal death and insulin-like growth factor I protection. JBC. 2002:277:17649–17656. 10.1074/jbc.M111704200 [DOI] [PubMed] [Google Scholar]
- 14. Saxena M, Dubey R. Target enzyme in Alzheimer’s disease: acetylcholinesterase inhibitors. Curr Top Med Chem. 2019:19:264–275. 10.2174/1568026619666190128125912 [DOI] [PubMed] [Google Scholar]
- 15. So WW, Liu WN, Leung KN. Omega-3 polyunsaturated fatty acids trigger cell cycle arrest and induce apoptosis in human neuroblastoma LA-N-1 cells. Nutrients. 2015:7:6956–6973. 10.3390/nu7085319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Braz-De-Melo HA et al. Potential neuroprotective and anti-inflammatory effects provided by omega-3 (DHA) against Zika virus infection in human SH-SY5Y cells. Sci Rep. 2019:9:20119. 10.1038/s41598-019-56556-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alessandri JM et al. Estradiol favors the formation of eicosapentaenoic acid (20: 5n-3) and n-3 docosapentaenoic acid (22: 5n-3) from alpha-linolenic acid (18: 3n-3) in SH-SY5Y neuroblastoma cells. Lipids. 2008:43:19–28. 10.1007/s11745-007-3117-6 [DOI] [PubMed] [Google Scholar]
- 18. Luchtman DW, Meng Q, Wang X, Shao D, Song C. Omega-3 fatty acid eicospentaenoic acid attenuates MPP+-induced neurodegeneration in fully differentiated human SH-SY 5Y and primary mesencephalic cells. J Neurochem. 2013:124:855–868. 10.1111/jnc.12068 [DOI] [PubMed] [Google Scholar]
- 19. Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer's disease mouse model. Nutr Health. 2006:18:249–259. 10.1177/026010600601800307 [DOI] [PubMed] [Google Scholar]
- 20. Freund-Levi Y et al. ω-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006:63:1402–1408. 10.1001/archneur.63.10.1402 [DOI] [PubMed] [Google Scholar]
- 21. Amtul Z, Uhrig M, Wang L, Rozmahel RF, Beyreuther K. Detrimental effects of arachidonic acid and its metabolites in cellular and mouse models of Alzheimer's disease: structural insight. Neurobiol Aging. 2012:33:831.e21–831.e31. 10.1016/j.neurobiolaging.2011.07.014 [DOI] [PubMed] [Google Scholar]
- 22. Hennig B, Toborek M, McClain CJ. High-energy diets, fatty acids and endothelial cell function: implications for atherosclerosis. J Am Coll Nutr. 2001:20:97–105. 10.1080/07315724.2001.10719021 [DOI] [PubMed] [Google Scholar]
- 23. Lu X, Yu H, Ma Q, Shen S, Das UN. Linoleic acid suppresses colorectal cancer cell growth by inducing oxidant stress and mitochondrial dysfunction. Lipids Health Dis. 2010:9:1–11. 10.1186/1476-511X-9-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002:56:365–379. 10.1016/S0753-3322(02)00253-6 [DOI] [PubMed] [Google Scholar]
- 25. Muthaiyah B, Essa MM, ChauhanV CA. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem Res. 2011:36:2096–2103. 10.1007/s11064-011-0533-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim SJ et al. Omega-3 and omega-6 fatty acids suppress ER-and oxidative stress in cultured neurons and neuronal progenitor cells from mice lacking PPT1. Neurosci Lett. 2010:479:292–296. 10.1016/j.neulet.2010.05.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taha A, Sharifpanah F, Wartenberg M, Sauer H. (). Omega-3 and Omega-6 polyunsaturated fatty acids stimulate vascular differentiation of mouse embryonic stem cells. J Cell Physiol. 2020:235:7094–7106. 10.1002/jcp.29606 [DOI] [PubMed] [Google Scholar]
- 28. Stanley WC, Khairallah RJ, Dabkowski ER. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2012:15:122–126. 10.1097/MCO.0b013e32834fdaf7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pantzaris MC, Loukaides GN, Ntzani EE, Patrikios IS. A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open. 2013:3:e002170. 10.1136/bmjopen-2012-002170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jayathilake AG, Kadife E, Luwor RB, Nurgali K, Su XQ. Krill oil extract suppresses the proliferation of colorectal cancer cells through activation of caspase 3/9. Nutr Metab. 2019:16:1–15. 10.1186/s12986-019-0382-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghazali R et al. High omega arachidonic acid/docosahexaenoic acid ratio induces mitochondrial dysfunction and altered lipid metabolism in human hepatoma cells. World J Hepatol. 2020:12:84–98. 10.4254/wjh.v12.i3.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hong MY et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002:23:1919–1926. 10.1093/carcin/23.11.1919 [DOI] [PubMed] [Google Scholar]
- 33. Li G et al. Antioxidant activity of docosahexaenoic acid (DHA) and its regulatory roles in mitochondria. J Agric Food Chem. 2021:69:1647–1655. 10.1021/acs.jafc.0c07751 [DOI] [PubMed] [Google Scholar]
- 34. Oppedisano F et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: their role in cardiovascular protection. Biomedicines. 2020:8:306. 10.3390/biomedicines8090306 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed during this study are available from the corresponding author upon reasonable request.