Abstract
Orthopedic disorders affecting bones, joints, muscles, tendons, and other tissues are prevalent among outpatients, often caused by trauma, sports, or tumor removal. Surgical intervention is common but may yield unsatisfactory results due to limited regenerative capacity and poor blood supply. Platelet-rich plasma (PRP), an autologous biocomponent, has been clinically applied in tissue regeneration and repair, yet it faces challenges such as unclear mechanisms, side effects, and uncontrollable release. This review provides evidence for further clinical research on PRP and its associated drug delivery strategies in orthopedics. We searched multiple databases, including PubMed, Embase, Scopus, and Google Scholar databases Inclusion criteria focused on original studies containing the phrases (“orthopedic injuries,” “nanotechnology,” “microsphere,” or “drug delivery system”) and (“platelet-rich plasma”) over two decades to provide evidence to support further clinical research on PRP combined with nanotechnology in osteoarthritis, fractures, cartilage repair, and other orthopedic fields. Excluding criteria were referred to studies only describing “nanotechnology,” “microsphere,” or “drug delivery system”. In conclusion, PRP is a novel therapeutic tool for orthopedic diseases with advantages over traditional surgery. However, its clinical efficacy, action mechanisms, and preparation standards need further clarification. Future research should optimize PRP's therapeutic concentration, administration, and timing, and explore high-concentration PRP GFs as alternatives.
Keywords: Platelet-rich plasma, Growth factors, Cytokines, Nanotechnology, Orthopedic injuries
Graphical abstract
Illustration of nanoparticles-mediated platelet-rich plasma in treating orthopedic injuries. Platelet-rich plasma can be delivered with drug delivery systems (DDS).
1. Introduction
Orthopedic disorders impact bones, joints, muscles, tendons, and other tissues. Orthopedic injuries are prevalent among outpatients and are typically caused by trauma, sports-related activities, or tumor removal. Generally, surgical intervention is necessary to treat the injuries. However, surgical intervention modalities frequently lead to unsatisfactory results. The limited regenerative capacity and inadequate blood supply may make the defect area difficult to heal (Fang et al., 2020). Furthermore, orthopedic injuries may result in osteoarthritis (OA) formation due to pain and activity limitations (Di Martino et al., 2019; Liu et al., 2017). Therefore, an efficacious treatment strategy is imperative to preserve joint function and alleviate pain, thereby enhancing the quality of patients. Platelet-rich plasma (PRP), an autologous bio-component prepared from a patient's blood, boasts a higher concentration of platelets. The cytokines and growth factors (GFs) in the platelets can repair the damaged tissues of tendons, ligaments, and cartilage by maintaining the stability of the intra-articular environment (Kawabata et al., 2023). In addition, PRP has been extensively utilized clinically for tissue regeneration and repair, particularly in orthopedics, due to the capability of modifying the microenvironments of lesion sites and stimulating tissue restoration and physiologic healing (Zhang et al., 2022a). Meanwhile, autologous PRP minimizes the risk of immune rejection (Manole et al., 2024). However, several challenges remain regarding the applications of autologous PRP. The mechanisms by which PRP works in the human body and the possible side effects still need to be clarified through extensive research. Issues such as uncontrollable release, instability, off-target delivery, and below-effect-acting concentration have also been noted and discussed.
In this context, drug delivery systems (DDSs) offer some promising choices for solving PRP therapy-facing issues. The shape and size of materials can be meticulously regulated by the microencapsulation process or nanotechnology so that these carriers have similar structures to biological molecules and vesicles and can be designed with various functions (Kim et al., 2010). Incorporating drugs into microscale or nanoscale particles (MPs or NPs) may enhance efficacy, prolong half-life, and improve specific binding properties (Pistone et al., 2017). These technologies have also been widely used in orthopedics, including diagnostic modalities, targeted drug delivery, vertebral disk regeneration, and implantable materials (Pleshko et al., 2012). Particles can encapsulate drugs steadily, control drug release, prolong retention time, and improve the drug's pharmacodynamics in vivo (Barani et al., 2023; Smith et al., 2018). Meanwhile, the rational design of particles, especially NPs, helps the drug diffuse and penetrate the extracellular matrix (ECM) and joint tissues to promote cartilage repair (Wen et al., 2023). Combining PRP with nanoscale DDSs aims to address the limitations of PRP therapy and enhance its safety and efficacy in disease treatment through precise delivery, long-term release, and improved stability of PRP. We reviewed PRP's application in orthopedics over the past two decades, focusing on studies that combine PRP with DDSs. The aim is to provide evidence for further clinical research on PRP and its associated DDSs in osteoarthritis, fractures, cartilage repair, and other orthopedic areas (Fig. 1).
Fig. 1.
The applications of nanoparticles mediated platelet-rich (PRP) plasma in the orthopedic field. PRP can be delivered with DDSs such as microspheres, extracellular vesicles, hydrogel particles, and polymeric nanoparticles, by Figdraw (www.figdraw.com).
2. Method
The research followed PRISMA 2020 guidelines to ensure a comprehensive and systematic review, enhancing credibility and accessibility for future researchers (Page et al., 2021). Specific criteria ensured high-quality, original research articles on “nanotechnology,” “microsphere,” or “drug delivery system” of PRP for orthopedic injuries were included, focusing on peer-reviewed English studies over two decades, while excluding studies only describing “nanotechnology,” “microsphere,” or “drug delivery system”. The patents were also excluded. The databases included PubMed, Embase, Scopus, and Google Scholar. All search results were exported to Mendeley for duplicate removal, followed by a two-stage filtering: title relevance screening and detailed abstract/complete text examination. Reviewers ensured articles met inclusion criteria before data extraction, which was organized into tables by factors like soft or complex type of nanocarriers, disease class, in vitro or in vivo outcomes of the combination of PRP and DDS.
3. Platelet-rich Plasma (PRP)
3.1. Concept of PRP
PRP is an autologous biological component obtained from a patient's blood via centrifugation (Chueh et al., 2022; Everts et al., 2020). PRP was first defined in hematology in the 1970s when hematologists referred to the supraphysiologic concentration of platelets in plasma as PRP. Evidence suggests that PRP was used primarily to treat patients with thrombocytopenia (Gupta et al., 2021; LaBelle and Marcus, 2020). In 1982, Childs CB et al. (Childs et al., 1982) discovered that plasma containing platelet-derived GFs can promote cell growth. Gibble JW et al. (Gibble and Ness, 1990) summarized platelets' hemostatic and adhesive properties and delved into platelet-related applications in the surgical field. Since then, PRP research has grown rapidly. Nowadays, it has been shown that PRP has anti-inflammatory and analgesic properties, along with the capability to improve tissue regeneration, etc. Additionally, PRP plays an essential role in various clinical disciplines, including orthopedics, burns and plastic surgery, and neurosurgery (Liang et al., 2022). Although PRP has been extensively studied in recent decades, the lack of consensus terminology, classification, and characterization has led to confusion about the terms associated with PRP. Over the years, different classification systems have been proposed to define various categories of PRP, as shown in Table 1. In 2016, Magalon et al. (Magalon et al., 2016) introduced the DEPA classification according to Dose, Efficiency, Purity, and Activation of PRP. Therefore, the quantity of platelets in the PRP, the purity of the product, and platelet status before injection become the focus of attention (Alves and Grimalt, 2017). In addition, based on PRP fibrin structure and platelet content, PRP is categorized into four primary groups: leucocyte- and platelet-rich fibrin (L-PRF) and pure platelet-rich fibrin (P-PRF), pure PRP (P-PRP) with lower content of leukocyte, and leucocyte- and platelet-rich plasma (L-PRP). Such a categorization takes into account the different biological characteristics and mechanisms of PRPs, which differ markedly in their clinical application. This categorization can be used further to investigate the impact of these products (Dohan Ehrenfest et al., 2014).
Table 1.
Classification systems of PRP.
| Classification systems | Criteria | Types of PRP | Features | Refs. |
|---|---|---|---|---|
| Dohan Ehrenfest classification | Based on the leukocyte enumeration and fibrin framework | P-PRP | The first classification system for platelets | (Dohan Ehrenfest et al., 2009) |
| L-PRP | ||||
| P-PRF | ||||
| L-PRF | ||||
| Mishra's classification | Based on leukocyte concentrations, platelets and their activation status | L-PRP solution | A specialized classification of PRP has been proposed in sports medicine. | (Mishra et al., 2012) |
| L-PRP gel | ||||
| P-PRP solution | ||||
| P-PRP gel | ||||
| PAW classification | Based on the number of platelets, the method of platelet activation, and the existence of leukocytes | “P3-x-Aα” represents three parameters: Platelet levels (cell numbers/μL): (1) > 1,250,000; (2) > 750,000 -1,250,000; > baseline - 750,000; (4) ≤ baseline |
Limited as it encompasses solely the PRP family | (DeLong et al., 2012) |
| X-exogenous activation | ||||
| Existence of WBCs: above the baseline (a) or at/below the baseline (b) | ||||
| Existence of neutrophils; exceed the baseline (i) or remain at or below the baseline (ii) | ||||
| PLRA classification | Based on the concentration of platelet (cells/μL), the presence of leucocytes (<1% – negative or > 1 % - positive), the proportion of neutrophils, the level of RBCs (<1 % - negative or > 1 % - positive), and the state of activation (no - negative or yes - positive) | The first classification system that proposed the inclusion of RBC concentration as a variable | (Mautner et al., 2015) | |
| DEPA classification | Based on injected dose, efficiency, purity, and activation status | Efficiency gauged by the rate of platelet recovery: > 5 (a), 3–5 (b), 1–3 (c), <1 (d) |
New classification systems | (Magalon et al., 2016) |
| Purity assessed through the relative composition of platelets: > 90 % (a), 70–90 % (b), 30–70 % (c), <30 % (d) |
||||
| The activation status: >90 % (a), 70–90 % (b), 30–70 % (c), <30 % (d) | ||||
| MARSPILL classification | Based on the preparation technique, spin number, imaging guidance, and luminescent activation | M (Method), A (Activation-), R (Red blood cells-P), S (Spin 2), P (Platelets [4–6]), I (G+) (Image guidance), L (Leukocytes-R [2–3]), L (Light activation) |
This classification also includes PRP's preparation, composition, and application process. | (Lana et al., 2017) |
| Platelet Physiology Subcommittee classification | Based on the ratio of leukocyte to RBC activation process, platelet levels, and the preparation methods | PRP | The classification system encompassed all principal factors of PRP production; however, the expansive range of platelet concentration resulted in a lack of precision. | (Harrison, 2018) |
| Red cell-rich PRP | ||||
| L-PRP | ||||
| red cell and leukocyte-rich PRP |
3.2. Composition and preparation of PRP
PRP is the plasma of autologous blood from whole blood, enriched with a higher concentration of platelets, 4–8 times higher than normal platelets. Platelets have been divided into three primary types of secretory granules: lysosomes, α-granules, and dense γ-granules (Mumford et al., 2015). Moreover, it also contains chemokines and proteins (Thu, 2022). Studies have shown that the α-granules can release various GFs and cytokines. Cytokines and GFs are crucial in tissue healing, effectively promoting tissue repair (Table 2). They have the functions of cell proliferation stimulants, cell migration chemoattractants, and mitogens (Harrison et al., 2011). Table 2 summarizes the types and functions of GFs and cytokines in PRPs. Among them, the GFs mainly include vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor (IGF), transforming growth factor (TGF), and platelet-derived growth factor (PDGF) (Cecerska Heryć et al., 2022). Multiple studies have shown that the GFs derived from platelets in PRP can stimulate the growth of newly formed blood vessels and enhance metabolism at the injection site. Therefore, the PRP treatment can promote the regeneration and reconstruction of tissue (Fang et al., 2020).
Table 2.
Major cytokines and GFs in PRP.
| Names | Features | Refs. | |
|---|---|---|---|
| GFs | VEGF | 1. Promotes angiogenesis | (Gobbi and Vitale, 2012; Rodrigues et al., 2019; Street et al., 2002) |
| 2. Anti-apoptotic effect | |||
| Fibroblast growth factor (FGF)-2 | 1. Regulates the proliferation and migration of a wide range of cells | (Gobbi and Vitale, 2012) | |
| 2. Accelerated tissue regeneration and repair | |||
| PDGF | 1. Accelerates cell proliferation and tissue repair | (Gobbi and Vitale, 2012; Hollinger et al., 2008) | |
| TGF-β | 1. Regulates cell functions, including suppression of cell proliferation and promotion of cell differentiation | (Gobbi and Vitale, 2012; Komaki et al., 2006) | |
| IGF | 1. Regulates cell proliferation, differentiation and metabolism | (Middleton et al., 2012; Sample et al., 2018) | |
| EGF | 1. Promotes skin tissue repair and soft tissue regeneration, thus accelerating routine healing | (Martínez et al., 2015; Mussano et al., 2016) | |
| Tumor necrosis factor (TNF)-α | 1. Activation of nuclear factor kappa B (NF-kB) signaling pathway | (Bendinelli et al., 2010; Riboh et al., 2016) | |
| 2. Exerts a pro-inflammatory response and anti-apoptosis | |||
| Cytokines | IL-1b | 1. Regulation of NF-kB signaling pathway | (Hudgens et al., 2016) |
| 2. Modulation of inflammatory and immune responses | |||
| Matrix metalloproteinase-9 | 1. Degradation of ECM molecules and collagen | (Liu et al., 2009; Oh et al., 2015) | |
The preparation of PRP usually includes the double-centrifugation method and single-centrifugation method. The double-centrifugation method usually consists of soft spinning and hard spinning steps, whereas the single centrifugation method only needs one centrifugation separation process (Saqlain et al., 2023) (Fig. 2). The advantages of single-centrifugation include fast and efficient performance, simple operation, and cost-effective savings. Comparatively, the samples prepared by the double-centrifugation method have better platelet enrichment, greater compliance with sterility, and less probability of cell contamination. Until now, there have been no standardized procedures for the blood samples and preparation processes, and the quality and effects of PRP have varied. Studies have shown that factors such as the amount of whole blood, rotation rate, platelet agonist, and preparation temperature can affect the quality and effects of PRP. Sabarish et al. (Sabarish et al., 2015) investigated the impact of rotation rates (1000–3600 rpm) and centrifugation time (4 to 15 min) on PRP quality, respectively. The results demonstrated that lower rotation rates and less time can obtain higher platelet content and enrichment rates. Additionally, higher rotation rates may result in platelet aggregation or disintegration. Lansdown et al. (Lansdown and Fortier, 2017) emphasized that factors related to patients also influenced platelet concentration. Meanwhile, both the feeding and the time of the blooding sample affect the final quality of PRP.
Fig. 2.
Preparation of platelet-rich plasma by centrifugal methods. By Figdraw (www.figdraw.com).
The standard protocol for preparing PRP is crucial for the quality control of the obtained PRP and is also a prerequisite for evaluating the results of clinical experiments (Lin et al., 2021). However, in the case of orthopedics, Chahla et al. found that only 11 of the studies provided preparation protocols for PRP, and only 17 studies provided qualitative indications of PRP compositions (Chahla et al., 2017). Therefore, the current studies must improve the transparency of the clinical and laboratory reports on PRP to promote standardization of PRP production and ensure the safety of use (BW et al., 2019).
3.3. Biological properties and action mechanisms of PRP
PRP includes numerous GFs, cytokines, and differentiated adhesion molecules, which trigger the activation and proliferation of mesenchymal stem cells (MSCs), fibroblasts, neutrophils, and smooth muscle cells (Zahn et al., 2017). Cytokines and adhesion molecules in PRP can promote migration, adhesion, differentiation, and proliferation of cells. For instance, the activated stromal cell-derived factor (SDF) 1α mediates cell migration and homing to the repair site. Some GFs, such as PDGF, TGF, VEGF, etc., play an essential role in promoting collagen synthesis (T et al., 1999), osteoblast proliferation (Shimoaka et al., 2002), macrophage activation, angiogenesis (Murakami et al., 2008), chemotaxis of immune cells and fibroblast (Seghezzi et al., 1998), migration and mitosis of endothelial cells (Lee et al., 2010), cytokine secretion from epithelial and mesenchymal cells (Seckin et al., 2022), and differentiation of epithelial cells (Murakami et al., 2008). Each factor plays a different role in specific tissues. For instance, PDGF is a critical cytokine in the early stages of tissue healing, promoting cytokinesis and matrix production. TGF-β regulates collagen and proteoglycan synthesis and modulates the release of other GFs. VEGF is a signaling protein that promotes neointima formation and vascular system development, and its interactions with the surface receptors of endothelial cells stimulate the migration and mitosis of endothelial cells. Hepatocyte growth factor (HGF) synergizes with VEGF to promote vascularization; IGF plays a vital role in tissue repair, maturation, and remodeling; and EGF promotes cell growth, proliferation, and differentiation through its interactions with the EGF receptor (B et al., 2013; Manole et al., 2024). In addition, fibronectin is also an important component of PRP that produces a suitable three-dimensional (3D) structure that provides a good scaffold for cell repair, facilitates the secretion of GFs and tissue repair, shrinks the wound to promote coagulation and wound closure, and stimulates tissue regeneration (Wang et al., 2023).
PRP is a key factor in triggering tissue repair and regeneration. There are four primary platelet activation methods: autologous thrombin, a combination of thrombin and CaCl2, a mixture of 10 % type I collagen and CaCl2, and the freeze-thaw method. Different activation methods determine the amount and kinetics of GFs released from PRP (Fang et al., 2020). For example, studies showed that the GFs released from PRP were increased gradually at 15 min and up to the peaks at 24 h by the CaCl2-activated method (Cavallo et al., 2016). In contrast, the GFs released from PRP activated by autologous thrombin were less effective and induced less platelet aggregation. In addition, CaCl2-activated PRP was significantly better than the other methods in releasing PDGF (Textor and Tablin, 2012). Therefore, choosing the appropriate activator and standardizing the platelet activation process are critical steps to optimize the release of various GFs and cytokines during application.
3.4. Clinical applications of PRP in orthopedics
3.4.1. Regenerative medicine
In the emerging field of tissue engineering, PRP is widely applied as a novel therapeutic tool in cardiothoracic surgery, dentistry, wound healing, orthopedics, dermatology, plastic surgery, hair loss, etc. (Seckin et al., 2022). The following contents summarize the advances of PRP in the field of orthopedics. In animal experiments, Takahiro et al. explored the effects of intravertebral disc injection of PRP releaser (PRPr) in rabbits on condylase-induced degenerative intervertebral disc (IDV) regeneration. The data suggested that the group injected with PRP had statistically lower histologic scores than the other group (p < 0.01). It was demonstrated that PRPr promoted the regeneration of condylase-induced rabbit IVD (Hasegawa et al., 2023). The data suggested that PRPr injection therapy may be suitable for patients with herniated discs who have poorly recovered from disc degeneration induced by condolence. In an animal model of hindlimb ischemia, Stilhano et al. (Stilhano et al., 2021) investigated the mechanisms of L-PRP and pure P-PRP promoting the regeneration of ischemic skeletal muscle of mice. The results showed that 1 % of PRPs induced higher metabolism and increased survival in C2C12 and NIH3T3 cells. Ischemic limbs treated with PRP increase ischemic mice's skeletal muscle mass and strength. Autologous PRP dramatically minimizes the risk of allergic reactions or incompatibility. It has been shown that PRP holds broad promise in wound regeneration by enhancing collagen synthesis and remodeling (Manole et al., 2024). In a recent article by Sharun et al. (Sharun et al., 2024), the potential of PRP for treating tendon and ligament injuries, OAs, and fractures in experimental studies using goats and sheep was reported. Animal-derived PRP studies provide strong evidence for the application of PRP in biological repair and regenerative medicine.
3.4.2. Anti-inflammatory and analgesic effects
Tohidnezhad et al. (Tohidnezhad et al., 2017) demonstrated that PRP exerted anti-inflammatory effects through cytokines, including IL-1β and TNF-α, that can reduce the inflammatory responses of human synoviocytes under inflammatory conditions. In treating chronic patellar tendinitis, PRP injections show better pain relief and functional recovery compared to conventional extracorporeal shock wave therapy (ESWT) (Smith and Sellon, 2014). A systematic evaluation showed that PRP injections were used to promote healing at the interface of tendon and bone. The injections of PRP for arthroscopic rotator cuff repairs may reduce postoperative pain and facilitate functional recovery (Samy et al., 2020). Bohren et al. reviewed the potential of PRP injection in neuropathic pains (Bohren et al., 2022). The anti-inflammatory mediators released from PRP can reduce inflammation and pain.
3.4.3. Fighting infections
Chronic infections are a clinical challenge to disease prevention, treatment, and management. Autologous PRP minimizes the risk of immune response and pathogen transmission (Jones et al., 2018). Also, after PRP is activated, many bioactive molecules, such as anti-microbial proteins, cytokines, and the released GFs, can inhibit bacteria and help wound healing. Badade et al. confirmed that PRP in vitro inhibited the adhesion and growth of Porphyromonas gingivalis and Porphyromonas actinomycetes to form Actinobacteria aggregates (periodontal pathogens), thus protecting the gingiva (Badade et al., 2016). Cervelli et al. investigated a new clinical choice to treat complex wounds with bone exposure after lower extremity surgery under PRP with HA adjuvant. The study involved 15 patients, all of whom suffered from bone-exposed wounds following lower extremity trauma. The data confirmed that the mean time of re-epithelialization of 73.3 % of patients who received the combination treatment of PRP and HA was 8.1 weeks. Comparatively, only 30 % of individuals who used only HA dressings realized the same responses (Cervelli et al., 2011). Rahimi et al. (Rahimi et al., 2023) assessed the therapy effects of allogeneic PRP, fibrin glue, and collagen matrix in treating severe limb-threatening open tibial fractures. The results showed that after treatment, the patient's wound closed completely, formed tissue granules, and successfully preserved his right leg. This suggests that combining these components can decrease the odds of infection, synergistically accelerate wound healing, and protect the limb. PRP is used to prevent postoperative infections and treat chronic wounds or bone infections. However, a significant and compelling clinical rationale is still needed to support this (Zhang et al., 2019b).
3.5. Limitations
PRP has been extensively selected for clinical application and has become the “superstar” of orthopedic treatments. However, the adequate time of PRP in the human body is short due to the short half-life of cytokines and GDs. Compared to antibiotics, PRP's antimicrobial effects are shorter and weaker (Mouanness et al., 2021; Zhang et al., 2019b). In addition, PRP treatment has lower specificity, weaker stability, and difficulty in controlling local concentrations. The standardized PRP preparation must provide reliable quality assurance for basic research and clinical applications. However, the current PRP preparation techniques lack a unified standard, compromising the quality of PRP products. Therefore, establishing expert consensus on the standardization of PRP preparation techniques and quality control is essential for ensuring the safety and efficacy of PRP in clinical use. Indeed, the quality and composition of autologous biocomponents may change with changes in the patient's condition, thus increasing the difficulty in standard setting. Hopefully, the black box of practical components must be fully elucidated to formulate artificial “PRP” based on effect ingredient formulations.
4. PRP-contained drug delivery systems
DDSs can achieve localized higher drug concentrations in the target tissue and reduced side effects in other tissues by controlling drug release. This is particularly crucial in tissues with limited vascularization, such as osseous tissue. In current clinical research, NPs have been becoming a novel means of delivery of PRP to improve the efficacy of active ingredients. Delivery of active ingredients in PRP using carriers such as exosomes, microvesicles, microspheres, polymeric NPs, etc., can bring a series of potential advantages. The following contents describe the progress of PRP combined with NPs in orthopedics (Table 3).
Table 3.
PRP-related DDSs.
| DDSs | Applications | Targets | Advantages | Refs |
|---|---|---|---|---|
| PRP-Exos | Rotator cuff tendon-bone healing; Femoral head osteonecrosis (FHON) |
Enhance tendon-bone healing; block apoptosis through Akt/Bad/Bcl-2 pathway | Accelerates tissue healing; reduces apoptosis effectively | (Han et al., 2024; Tao et al., 2017) |
| PRP-Exos-Gel | Subtalar osteoarthritis (STOA) | Prolong PRP release, inhibit chondrocyte apoptosis, and recruit stem cells | Increased local retention time, enhanced therapeutic efficacy | (Zhang et al., 2022a) |
| Chitosan-gelatin/nano-hydroxyapatite (CS-G/nHA) |
Osteogenic differentiation | Enhance osteoblast differentiation and mineralization | Improves scaffold integration, enhances osteogenic potential | (Sadeghinia et al., 2019) |
| Chitosan nanocomposite membrane | Burn wound healing | Regulate gene expression in wound healing | Enhanced therapeutic outcomes in burn management | (He et al., 2022; Tramś et al., 2022) |
| HA hydrogel | Cartilage defect repair | Facilitate cartilage regeneration | Injectable, supports significant cartilage regeneration | (Yan et al., 2020) |
| GelMA/Chitosan microcarriers (IGMs) |
Dermal papilla cell bioactivity enhancement | Sustain release of GFs; enhance bioactivity of dermal cells | Mimics ECM environment sustains cellular bioactivity | (Zhang et al., 2022b) |
| Hydroxyapatite-collagen scaffold | Osteochondral defect repair | Promote cartilage and bone regeneration | Enhanced bone regeneration, but interaction with PRP requires optimization. | (Kon et al., 2010) |
4.1. Extracellular vesicles
In recent years, increasing research has focused on extracellular vesicles (EVs) derived from PRP. The EVs usually refer to nano-sized structures with a lipid bilayer that does not replicate (Antich Rosselló et al., 2021; Wu et al., 2021). In a study by Anitua et al. (Anitua et al., 2023), the signs of progress of PRP-EVs in regenerative medicine were comprehensively reviewed, demonstrating that PRP-EVs represent a promising treatment approach for tissue restoration and regeneration. Besides this, PRP-EVs can promote cellular proliferation, migration, and angiogenesis (Dai et al., 2023; Rui et al., 2021; Tao et al., 2017; Zhang et al., 2022a) but also reduce the inflammatory response, apoptosis and oxidative stress, etc. (Dai et al., 2023; Otahal et al., 2021; Xu et al., 2021a; Zhang et al., 2022a). In addition, due to the lower immunogenicity of PRP, its ability to protect against degradation, and its capability to overcome biological barriers, PRP-EVs can function at the molecular level through various signaling pathways. These signaling pathways encompass Akt/Bad/Bcl-2 (Tao et al., 2017), Wnt/β-catenin (Dong et al., 2022; Liu et al., 2019), YAP (Guo et al., 2017), TLR4 (Zhang et al., 2019a), PI3K/Akt (Zhang et al., 2020), PGC1α-TFAM (Dai et al., 2023), Keap1-Nrf2 (Xu et al., 2021a), and AKT/ERK (Rui et al., 2021). Table 4 summarizes these signaling pathways.
Table 4.
Mechanisms of action of PRP-derived vehicles related to the signaling pathway.
| Signaling pathways | PRP-derived vehicles | Diseases | Action mechanisms | Refs |
|---|---|---|---|---|
| Wnt/β-catenin | PRP-derived exosomes | Osteoarthritis (OA), Osteonecrosis of the femoral head (ONFH) | Exosomes derived from PRP stimulated the Wnt/β-catenin signaling pathway, enhancing chondrocyte growth and reducing cell death in osteoarthritis. In cases of osteonecrosis affecting the femoral head, they promoted the growth of osteoblasts and increased the expression of osteogenic markers, thus preventing bone degeneration caused by glucocorticoids. | (Dong et al., 2022; Liu et al., 2019; Tao et al., 2017) |
| AKT/ERK | PRP-derived exosomes | Tissue regeneration, Angiogenesis, Wound healing | Exosomes derived from PRP, particularly when activated with thrombin and calcium gluconate, greatly enhanced endothelial cells' proliferation, migration, and angiogenesis by triggering the AKT/ERK signaling pathway. The activated exosomes showed increased levels of angiogenic GFs like VEGF, PDGF-BB, bFGF, and TGF-β, which boosted their therapeutic effects for tissue regeneration and wound healing. | (Guo et al., 2017; Rui et al., 2021) |
| TLR4 | PRP-derived exosomes | Diabetic retinopathy | High glucose-induced PRP-derived exosomes activated the TLR4 signaling pathway in retinal endothelial cells, enhanced TLR4 and downstream protein expression, and markedly increased the release of the pro-inflammatory cytokine CXCL10, mediating retinal endothelial damage. | (Zhang et al., 2019a) |
| PI3K/Akt | PRP-derived exosomes | Retinal fibrogenesis, Müller cell fibrosis | Diabetes mellitus-derived PRP-exosomes activated the PI3K/Akt signaling pathway, significantly enhancing retinal Müller cell proliferation, migration, and the expression of fibrogenic markers, including connective tissue GFs (CTGF) and fibronectin, thereby promoting retinal fibrosis. | (Zhang et al., 2020) |
| SIRT1/PGC1α/TFAM | Platelet-derived extracellular vesicles | Intervertebral disc degeneration (IVD) | Platelet-derived extracellular vesicles (PEVs) restored compromised mitochondrial function, reduced oxidative stress, and reprogrammed cellular metabolism in nucleus pulposus cells through the modulation of the SIRT1/PGC1α/TFAM signaling pathway, thereby inhibiting apoptosis and senescence and delaying the progression of disc degeneration. | (Dai et al., 2023) |
| Keap1/Nrf2 | PRP-derived exosomes | IVD | Exosomes from PRP transported miR-141-3p into nucleus pulposus cells, targeting and breaking down Keap1 mRNA, which led to the liberation of Nrf2 from the Keap1-Nrf2 complex. Once activated, Nrf2 moved into the nucleus, boosting antioxidant defenses and lowering oxidative stress and pro-inflammatory cytokines, ultimately reducing disc cell apoptosis and degeneration. | (Xu et al., 2021a) |
| YAP | PRP-derived exosomes | Wound healing | Exosomes from PRP stimulated the YAP signaling pathway in keratinocytes, leading to increased cell proliferation, migration, and re-epithelialization, which helped speed up the healing of chronic skin wounds in diabetic models. | (Guo et al., 2017; Xu et al., 2021b) |
| Akt/Bad/Bcl-2 | PRP-derived exosomes | Steroid-caused osteonecrosis of the femoral head (ONFH) | Exosomes derived from PRP activated the Akt/Bad/Bcl-2 signaling pathway, which helped prevent apoptosis induced by glucocorticoids and encouraged the proliferation of osteoblasts. These effects helped maintain bone structure and halted the advancement of steroid-induced osteonecrosis. | (Tao et al., 2017) |
The cells release two subtypes of EVs, including microvesicles (MVs) and exosomes (Exos). They can be obtained by differential centrifugation (DC) (Xu et al., 2016). The Exos are nanosized EVs that were excreted from the endosomes of eukaryotic cells and were first discovered in 1983. Their sizes range from 40 to 160 nm in diameter, and the average size is about ∼100 nm. The biological formation of Exos begins with the double invagination of the plasma membrane, and then the intracellular multivesicular bodies are fused with the cell membrane. Afterward, they are released outside the cells (He et al., 2018; Kalluri and LeBleu, 2020; Lai et al., 2022). Exos help realize the communication between healthy and pathological cells and may be the best carriers for clinical therapeutics. It has a bilayer membrane structure that protects the active agents, prolongs the circulating half-life of the payloads, and enhances their bioactivity. Exos can also improve the natural components in the lipids and proteins of the carrier's bioactivity and reduce their adverse effects.
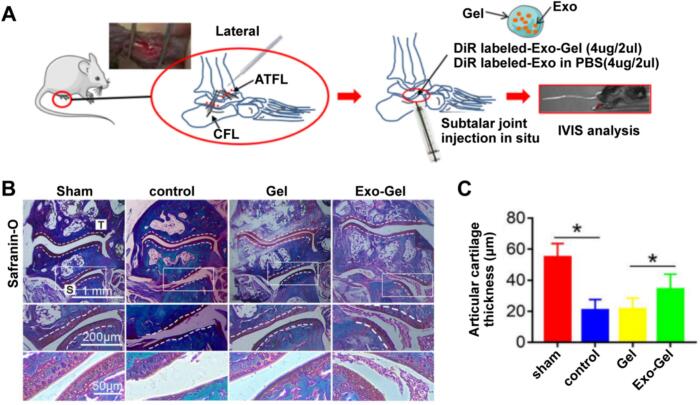
Meanwhile, Exos can carry many payloads, such as TGF β1, PDGF BB, VEGF, and so on, to avoid the degradation of these contents in vivo. Exos exhibit no species differences and do not induce immunogenicity. In addition, the signals carried by Exos can be transmitted across species (He et al., 2018; Lai et al., 2022; Zhang et al., 2022a). Exos can influence the immune response through antigen presentation, thus crucial in stimulating or inhibiting the immune system. Biological or chemical modification of the vesicular lipid bilayer can enhance the targeting ability of Exos (He et al., 2018). Exos can be distinguished from other vesicles by specific protein structures, including CD9, CD63, and CD81. Exos derived from PRP have also been used in clinical applications. Han et al. (Han et al., 2024) explored the effects of PRP-derived exosomes (PRP-Exos) on the healing of rotator cuff tendon (RCT)-bone. New Zealand rabbits were used to construct an RCT animal model, and HE staining was performed to observe the repair of tendon bone tissue. The data exhibited that the proliferation and differentiation of tendon-derived stem cells (TDSCs) were promoted due to PRP-Exos. The in vivo data have demonstrated that PRP-Exos has the potential to facilitate the prompt healing process of injured tendons. After being treated with PRP-Exos, rabbits showed better tissue alignment at the tear site and more substantial tendon bone healing. Zhang et al. (Zhang et al., 2022a) investigated whether PRP-Exo doped with thermosensitive hydrogel (Gel) could prolong the time of release within the joint and achieve better therapeutic effects in subtalar osteoarthritis (STOA) (Fig. 3A). The in vivo studies have demonstrated that Exo-gel can increase the local retention time of vesicles, inhibit the hypertrophy and apoptosis of chondrocytes, promote their growth, and may recruit stem cells, thereby delaying the onset of STOA (Fig. 3B-C). Therefore, clinical therapy with PRP-Exos combined with thermosensitive gel paves a new avenue for STOA. Tao et al. (Tao et al., 2017) hypothesized that PRP-Exos could trigger the Akt/Bad/Bcl-2 cascade reactions to block glucocorticoid (GC)-related endoplasmic reticulum (ER) stress-induced apoptosis due to the femoral head osteonecrosis (FHON) in rat. In their studies, the author created a dexamethasone-induced cellular model as well as a methylprednisolone (MPS)-induced rat model. The PRP-Exos was characterized, and the therapeutic responses of PRP-Exos on angiogenesis, proliferation, apoptosis, and osteogenesis in cells induced with GC were investigated (Fig. 4). In addition, they analyzed that the degree of GC-induced apoptosis was alleviated by PRP-Exos and identified the pathway of Akt/Bad/Bcl-2 to realize this alleviation. The data showed that PRP-Exos was able to block the apoptosis induced by GC in rats with the FHON due to the promotion of Bcl-2 expression through the Akt/Bad/Bcl-2 pathway, which was stimulated by endoplasmic reticulum stress.
Fig. 3.
Exo-Gel elongates the vesicle retention time in the defects of the ankle. (A) Scheme illustrating experimental design. After transecting of calcaneal fibular ligament/anterior talofibular ligament, the gel containing DiR-labeled Exo was injected into the defects of the subtalar joint to lead to the instability of the subtalar-ankle joint complex. (B) Histological staining using safranin-O was performed on the subtalar joint section to assess cartilage degeneration. The enlarged images below show the regions in the inset box. The thickness of the lateral calcaneus cartilage was gauged at 15 points in each section of B (Zhang et al., 2022a). Copyright © 2022, The Yu Zhang (s).
Fig. 4.
Impacts of PRP-Exos on cellular behavior and repair of the GC-triggered defected femoral heads of rats. (A) Ki67 immunostaining confirmed the early impacts of PRP-Exos on cell proliferation within the GC-triggered FHON tissue in rats. (B) TUNEL assay was used to analyze the early impacts of PRP-Exos on cell apoptosis within the GC-triggered FHON tissue in rats. (C) MicroFil was used to examine the long-term effects of PRP-Exos on the blood supply of the FHON tissue in rats. (D) Micro-CT scanning was used to observe the sagittal (SAG), transverse (TRA), and reconstructed coronal (COR) changes of the FHON bone after long-term PRP-Exos treatment as compared with the healthy or methylprednisolone (MPS) (Tao et al., 2017). Copyright © 2016, The Shi Cong Tao (s).
MVs formation requires rearranging molecules within the plasma membrane, leading to the changed composition of lipids and proteins (Ayers et al., 2015). One of the lipids enriched in MVs is cholesterol (42.5 %). Meanwhile, MVs contain proteins that usually undergo high post-translational modifications, including CD40, integrins, glycoprotein Ib, and P-selectin (Lai et al., 2022). Pharmacological inhibition experiments have shown that, after various stimuli, the enzyme aSMase is translocated to the plasma membrane to promote the formation of microvesicular particles (MVP) (Rohan et al., 2022). In addition, cytoskeletal elements and their regulators are also necessary for MVs biosynthesis (van Niel et al., 2018). Preparation methods for MVs include direct hydration, film hydration, and pH conversion methods (Fonseca et al., 2024). MVs diameters range from 50 to 1000 nm (van Niel et al., 2018). MVs released from cell surfaces are the markers of the apoptotic or activation state of the cells from which they originate, thus providing information on the nature and quantity of MVs in circulation as an aid to clinical diagnosis and treatment of tissue or organ status (Ayers et al., 2015). Effective identification of cell states by MVs can help to improve drug stability and realize controlled or sustained release of drugs, which shows massive promise within the realm of targeted drug delivery. MVs in the transport of bioactive substances can counteract the systemic immunosuppression induced by UVB radiation. UVB irradiation to human skin explants and the keratinocyte-derived human cell line HaCaT leads to platelet-activating factor receptor (PAFR)-dependent excretion of MVs (Liu et al., 2021). For the study of PRP-derived MVs, Lovisolo et al. (Lovisolo et al., 2020) investigated the wound-healing effects of PRP and PRP-derived MVs in an in vitro human keratinocyte model. They added PRP, activated calcimycin, and PRP-derived MVs separated by high-speed centrifugation to scratched keratinocyte monolayers, comparing the healing closure at 0 and 24 h. The results showed that PRP-derived MVs could fully replicate the wound-healing effects of PRP. This preliminary study suggested that using highly purified PRP-derived MVs obtained through cell sorting or ultracentrifugation may facilitate the application of PRP-derived MVs in animal models or clinical settings to further explore their therapeutic potential.
A recent report by Hou et al. (Hou et al., 2023) provided a systematic analysis of the roles of PRP-EVs in tissue regeneration. This article systematically examines the biological properties, extraction methods, identification processes, activation techniques, and preservation strategies associated with PRP-EVs. Furthermore, it explores their applications in OA and wound healing. Finally, the article underscores the significance of PRP-EVs in contemporary medicine and posits their potential as promising natural nanocarriers.
4.2. Microspheres
Microspheres are micron-sized spheres composed of a continuous phase of one or more mixed and dissolved polymers (Karan et al., 2020). Their distinctive spherical morphology and uniform size enable microspheres to adsorb or encapsulate ions, extracellular molecules, and drugs during tissue regeneration, making them ideal for drug storage and bioactive molecular delivery (Zhu et al., 2022). Current microsphere preparation methods include emulsion-based techniques, microfluidics, spray drying, co-precipitation, supercritical fluid processing, and superhydrophobic surface-mediated approaches (Li et al., 2024). Choi et al. (Choi et al., 2020) developed biodegradable polyethylene glycol (PEG) microspheres loaded with PRP using microfluidic technology. Their studies not only characterized the PRP-loaded PEG microspheres but also further determined whether platelet aggregation and clotting would affect microsphere degradation in the presence of PRP. The results showed that platelet aggregation could prolong the release of PRP from the microspheres. The microspheres show promise for controlled PRP release to accelerate tissue restoration and wound healing or inhibit tissue degradation in OA and IVD. In recent years, with the rapid development of hydrogel materials in the biomedical field, hydrogel microspheres are increasingly regarded as promising therapeutic carriers.
Researchers are now exploring their potential as injectable biomaterials for localized cell and drug delivery. For example, Yuan et al. (Yuan et al., 2024) developed GelMA hydrogel microspheres to modify the delivery kinetics of PRP, successfully extending its sustained release time. Experimental results showed that the hydrogel microspheres with sustained release features could enhance the effectiveness of PRP in treating endometrial lesions and achieving pregnancy. This study undoubtedly provides a new approach to managing endometrial disorders. Similarly, Zhou et al. (Zhou et al., 2021) immobilized PRP platelets onto gelatin microspheres (GMs), creating a biomimetic bioreactor (PRP + GMs) and exploring its wound-healing potential. Compared to PRP alone, PRP + GMs elongated and enhanced cytokines release, showing the potential to accelerate wound healing. The authors believe this gel microsphere is an injectable particle that can improve the therapeutic benefits of PRP.
Additionally, the application of hydrogel microspheres in orthopedics has gained attention. Nagae et al. (Nagae et al., 2007) successfully loaded PRP into hydrogel microspheres to treat IVD. The results showed that PRP was continuously released as the hydrogel degrades, suggesting that synergistic application of PRP and hydrogel microspheres may provide a new therapeutic approach for IVD. Lin et al. (Lin et al., 2024) summarized the relevant research and application of hydrogel microspheres as vehicles for cellular and pharmaceutical delivery in cartilage, bone, and soft tissue regeneration.
4.3. Polymeric nanoparticles
Polymeric nanoparticles (PNPs) are soft organic materials made of biocompatible lipids or polymers with particle sizes ranging from 1 to 1000 nm (Ong et al., 2021). Naturally synthesized PNPs are multifunctional nanocarriers with more features, including biodegradability, biocompatibility, and non-toxicity. As DDSs, PNPs are capable of controlling the rate of drug release, enhancing drug stability and bioavailability, and enabling drug delivery to desired tissues. As a result, the PNPs are now widely utilized in healthcare for various applications, such as prevention, diagnosis, and treatment of diseases (Swetledge et al., 2021). Loading an imaging substance or drug with solid or solution status into PNPs may improve diagnostic sensitivity or drug efficacy, elongate the half-life, and reduce side effects, enhancing patient safety and compliance (Patel et al., 2012). PNPs can deliver drugs via various routes, such as oral, intranasal, intravenous, or intraperitoneal administration (Zhang et al., 2021). Several techniques are available for preparing PNPs, including solvent evaporation, emulsification/solvent diffusion, emulsification/reversed salting out, and solvent substitution. The choice of method can be tailored based on the physicochemical characteristics of the drug encapsulated within the PNP and the specific requirements for the intended route of administration. Method I, the preparation process of solvent evaporation, is shown in Fig. 5A. The First step is to prepare an oil-in-water (o/w) emulsion, followed by the solidification process under vigorous stirring, and then harvest PNPs. Method II, the emulsification/solvent diffusion technique, is depicted in a schematic representation in Fig. 5B, allowing the formation of PNPs with sizes ranging from 80 to 900 nm due to the infiltration of organic solvent into the water phase. Method III, the emulsification/reversed salting out process, is idepicted in a schematic representation in Fig. 5C, which can be regarded as an improved emulsification/solvent diffusion process method. Adding salt into the water phase further decreases the solubility of drugs or particles, leading to a higher loading efficiency of active agents or recovery rate of PNPs. Method IV, the solvent substitution method, requires two miscible solvents (Fig. 5D) and the obtained NPs are typically characterized by well-defined sizes (Zielińska et al., 2020).
Fig. 5.
Different methods of preparing PNP (Zielińska et al., 2020) (A) Scheme of the solvent evaporation method. The first step is to emulsify the mixture to form an oil/water emulsion, then is a solidification step to remove the organ solvent under vigorous stirring, and the final step is to harvest PNPs. (B) Schematic representation of the emulsification/solvent diffusion method. In the emulsification/solvent diffusion process, PNPs with sizes ranging from 80 nm to 900 nm are prepared after solvent diffusion; (C) Schematic representation of the emulsification/reverse salting-out method. Adding salt further decreases the solubility of the particles or drug, thus increasing the recovery of PNPs or the loading efficiency of the drug; (D) Schematic illustration of the nanoprecipitation method. This method needs two miscible solvents and forms NPs typically of well-defined size. Copyright © 2020, The Aleksandra Zieli Ñska (s).
In addition, PNPs possess stimulus-responsive features that enable them to change their physicochemical properties under specific microenvironments in the body, such as temperature, electrolyte strength, pH value, etc. These features confirm that PNPs have an optimistic prospect for the clinical application of smart DDSs (Wang et al., 2024). In the future, PNPs will make more significant breakthroughs in the fields of precision medicine and drug delivery. Their potential mainly characterizes the application of PNPs in orthopedics as a biomaterial. It can be used as a scaffolding material to guide the regeneration and repair of bone tissue. For example, nanoscale hydroxyapatite gradient-coated materials have been shown to increase the shear strength of the implant-bone interface, thereby promoting early healing at the bone trauma interface (Hou et al., 2022). It can be hypothesized from clinical studies that the combination of PNPs and PRP will offer a more effective therapeutic option in orthopedic treatment. The GFs in PRP can promote the bioactivity of PNPs, while the physicochemical properties of PNPs can enhance the biocompatibility and functionality of PRP. PRP can be more precisely targeted to the damaged area delivered by PNPs, thereby accelerating bone repair (Kiaie et al., 2020). In addition, the PNPs consisting of PEG-PLGA nanoparticles (PLGA NPs) did not change the platelet reactivity, such as activation and aggregation of PRP, as well as internalized capabilities of resting or activated platelets (Bakhaidar et al., 2020). PLGA NPs solve the related safety problems caused by the effect of delivery carriers on platelet function.
4.4. Others
In addition, as a standard biomedical material (Wang et al., 2003; Williams et al., 2009), porous chitosan, hydrogel, membranes and nanocomposite scaffolds are also used as delivery systems to load PRP. Sadeghinia et al. (Sadeghinia et al., 2019) explored the impacts of activated PRP (a-PRP) and fibrin glue (FG) on the proliferation of human stem cells from dental pulp (h-DPSCs) and osteogenic differentiation of h-DPSCs. They then selected the scaffold made of chitosan-gelatin/nano-hydroxyapatite (CS-G/nHA) for its porous nature as the ideal base to seed h-DPSCs. The h-DPSCs were implanted on porous composite scaffolds of CS-G/nHA. Four scaffolds were prepared to culture h-DPSCs in the study: a-PRP/CS-G/nHA, a-PRP-FG/CS-G/nHA, FG/CS-G/nHA, and CS-G/nHA as a control. The results showed that the a-PRP-treated and FG-treated composite scaffolds preferentially displayed fibronectin networks on their surface, enhancing the mineralization of harvested cells and osteoblast differentiation.
Additionally, the a-PRP-FG/CS-G/nHA composite scaffolds enhanced the expression of bone marker genes from week 1 to week 3. He et al. (He et al., 2022; Tramś et al., 2022) investigated the combined effects of PRP plasma-containing gel with chitosan nanocomposite membrane laden with bone marrow stromal cells on the promotion of wound healing. They applied its impact to the repair of burns in specific locations, specialized burns, and burns of various ages. They found that the complex could modulate the expression of targeted genes and pathophysiological processes involved in wound healing. Yan et al. (Yan et al., 2020) tested the application potential of injectable HA hydrogel containing autologous PRP to repair the defected cartilage. The medial femoral condyle of porcine was impaired in forming focal cartilage defects of varying critical sizes (Fig. 6A). In a 6-month evaluation of a porcine model, it was discovered that HA hydrogel containing PRP has the potential to facilitate cartilage healing. This was evidenced in osteochondral defects that were 5 mm deep and 6.5 mm in diameter and full-thickness cartilage defects that measured 8.5 mm in diameter, showing significant signs of regeneration. Of course, studies surfaced that PRP may interfere with nanoparticle applications in orthopedics (Fig. 6B).
Fig. 6.
The influence of HA hydrogel containing PRP-treated for porcine cartilage regeneration (A) The surgical procedure of cartilage and osteochondral defects. Repaired tissue was stained with HE dyes to show the interface changes of osteochondral and cartilage and repaired area. (B) Typically, images of the appearance of the repaired tissue are used (Yan et al., 2020). Copyright © 2019, © The Yan, Wenqiang; Xu, Xingquan (s) 2019. Published by Oxford University Press. The injectable composite system of PRP/cell-loaded microcarrier/hydrogel. (C) Scheme of release test of the growth factor. Cumulative release of FGF and injecting PRP-laden DECHS into nude mice. (D) Expression of DP signatures of and morphological analysis of DPCs on IGMs: The images of DPCs marked with DAPI, Ki67, and Phalloidin (Zhang et al., 2022b). Copyright © 2022, The Yufan Zhang(s).
Zhang et al. (Zhang et al., 2022b) developed gelatin methacryloyl (GelMA)/chitosan-microcarriers (IGMs) containing PRP and seeded them with dermal papilla cells (DPCs) to imitate ECM. The IGMs with properties of appropriate swelling could sustain the release of GFs. Moreover, DPC/PRP-loaded IGMs were efficiently combined with epidermal cell (EPC)-loaded GelMA to create a PRP-laden DPC/EPC co-existed hydrogel system (DECHS). Afterward, this system was delivered to the subcutis of nude mice (Fig. 6C). The results have shown that the DECHS and IGMs effectively simulate a macro- and micro-environment that promotes DPC bioactivity (Fig. 6D). Kon et al. (Kon et al., 2010) added PRP to a novel multilayered gradient nanocomposite scaffold with hydroxyapatite NPs nucleating collagen fibers to investigate its role in the repair process of osteochondral defects in a sheep model. Animals were randomly grouped into the scaffold group, the scaffold containing PRP group, and the empty defect group (control). It was found that cartilage surface reconstruction and bone regeneration were significantly better in the scaffold-alone treatment group. Conversely, irregular cartilage surface integration and incomplete bone regeneration were observed in the PRP group. Cartilage and bone defects failed to heal for the control animals and were instead filled with fibrous tissue. Thus, this experimental surface hydroxyapatite-collagen scaffold promoted the repair of osteochondral lesions, but the addition of PRP interfered with the regeneration process and negatively affected the results. Therefore, the interactions of PRP with the components in the hydroxyapatite-collagen scaffold should be further clarified.
DDS development offers new PRP delivery strategies, but PRP formulation lacks a standard operating procedure (SOP), leading to variable platelet, WBC, RBC, and GF levels, which may affect clinical efficacy (Shome et al., 2024). Challenges also include DDS material selection and PRP loading and release mechanisms (Khandan-Nasab et al., 2025). Therefore, maintaining PRP component consistency and drug loading is crucial for clinical translation of PRP-contained DDS. Although there is currently no SOP for the preparation of PRP, there are various commercial PRP separation systems on the market that can achieve higher reproducibility in the preparation of PRP suspensions and reduce batch differences (Kevy and Jacobson, 2004; Lozada et al., 2001; Waters and Roberts, 2004). To control the content differences of PRP components, selecting single cytokine delivery that targets specific cell populations is a better choice to improve the quality of production, and also minimize delivery to non-targeted cells and tissues (Chen and Mooney, 2003; Khandan-Nasab et al., 2025). In addition, the development and application of microfluidics technology provides a solution for the robust production of DDS, by generating highly uniform carriers through fixed geometric shear fields and continuous processing (Rawas-Qalaji et al., 2023), thereby minimizing component fluctuations. These comprehensive methods ensure the key quality attributes of PRP-contained DDS and demonstrate the possibility of industrial expansion to some extent.
5. Clinical applications of PRP base on DDSs in orthopedics
5.1. Joint diseases
5.1.1. Osteoarthritis
Osteoarthritis (OA) hinders body movement and is a degenerative joint disease. Due to unsatisfactory biomechanics, OA often reduces quality of life (El-Kadiry et al., 2022). The common OA include temporomandibular joint (TMJ) OA and knee joint (KJ) OA. TMJ OA, an inflammatory disease of the TMJ and its surrounding tissues, is a low-grade, more severe temporomandibular disorder (TMD) (Lee et al., 2020). As a long-term degenerative disease, the pathologic features of TMJ OA are characterized by chondrocyte destruction, degradation of ECM and subchondral osteophytes, microcystic formation, osteoid formation, and concomitant synovial inflammation of varying degrees (Cardoneanu et al., 2023; Wu et al., 2022). The common symptoms include pain, stiffness, and the clicking sound that characterizes TMJ OA. Traditional surgical treatment methods may result in scarring, which can affect the appearance, and the facial nerve and parotid gland may also be affected. Thus, intra-articular injection is gradually gaining traction as a practical new approach to treat TMJ OA (Wu et al., 2022). The knee joint is a complex hinge joint composed of the distal femur, proximal tibia, patella, meniscus, free cartilage, ligaments, patellar fat pad, and synovium (Jang et al., 2021). The knee joint is also an essential tissue that stabilizes and supports the body in different postures. It has a motor function that allows people to perform some daily activities. Due to its high-frequency motion nature, the knee joint is prone to painful conditions, even developing knee osteoarthritis (KOA) (Gupta et al., 2021).
Meanwhile, trauma, aging, females, overweight, low bone density, muscle weakness, and joint laxity are also risk factors of KOA (Behzad, 2011). At present, the treatment of KOA mainly depends on physical therapy, such as physical factor therapy (shock wave therapy, infrared rays, iontophoresis, etc.), manipulative therapy (acupuncture, tuna, massage, etc.), and muscle-strengthening training (Lorenzo et al., 2021). Some medications (non-steroidal anti-inflammatory drugs, NSAIDs) or surgical interventions that include partial or total knee replacement and knee osteotomy (high tibial osteotomy or femoral osteotomy) can also be used in clinical for treating KOA (Szwedowski et al., 2021). Besides, intra-articular injection is also used in the management of KOA. Although intra-articular injection has better efficacy and fewer adverse effects compared with other treatments, there are some differences in the clinical impact of different injection drugs to treat OA.
Numerous novel treatments have been developed for OA, such as PRP, vitamin D, oral collagen, methylsulfonylmethane, and curcumin (Fuggle et al., 2020; Gupta et al., 2022). Among these, PRP has exhibited clinical benefits in OA patients. Research has shown that PRP primarily exerts anti-inflammatory and regenerative effects by releasing various GFs and cytokines. These GFs and cytokines mainly include PDGF, TGF, VEGF, EGF, FGF, CTGF, IGF, HGF, angiopoietin-1 (Ang-1), keratinocyte growth factor (KGF), platelet factor 4 (PF4), SDF, and TNF (Everts et al., 2020). Studies have identified the roles and mechanisms of high concentrations of GFs and cytokines in PRP within OA through both in vitro and in vivo animal experiments, suggesting that these GFs and cytokines can serve as commercial alternatives to PRP (Cai et al., 2023; Zhu et al., 2021). However, not all cytokines released by PRP are beneficial to OA. For example, VEGF, TNF-α, Ang-1, and SDF-1α may have negative impacts on OA (Table 5). Since these GFs, which may have a negative effect on PRP treatment of OA, limit the use of PRP in OA treatment, some studies have begun to try different strategies to avoid this negative effect. For example, Lee et al. (Lee et al., 2022) attempted to reduce unwanted biological activity by using GF-binding microspheres. They sequestered VEGF in PRP by using VEGF-binding microspheres, thereby developing a VEGF-attenuated PRP. Further research showed that the attenuation of VEGF in PRP not only did not inhibit the effects of PRP on the differentiation of stem cells for cartilage formation in vitro, but also significantly improved the healing of rat OA cartilage. Regarding the impact of TNF-α on the development of OA, studies have shown that in obese adults, serum TNF-α levels are elevated (Park et al., 2005) and obesity is positively correlated with the incidence of musculoskeletal diseases (Gnacińska et al., 2009). This research demonstrated a close relationship between obesity and OA (Xie and Chen, 2019). Therefore, when using PRP to treat OA, monitoring the TNF-α levels in PRP may be necessary.
Table 5.
Summary of cytokines in the PRP that are beneficial, detrimental, or unknown in OA treatment.
| Effects | Cytokines | Features | Refs |
|---|---|---|---|
| Beneficial | TGF-β | Reducing endogenous TGF-β led to less osteophyte formation and cartilage breakdown, while TGF-β aided in chondrocyte growth and cartilage preservation. | (Zhu et al., 2021) |
| TGF-β downregulated MAPK6 expression, leading to the polarization of M2 macrophages in the synovium. | (Bakker et al., 2001) | ||
| Exosomal miR-135b from TGF-β1-modified MSCs reduced cartilage damage by encouraging M2 synovial macrophage polarization through MAPK6 targeting. | (Wang and Xu, 2021) | ||
| PDGF | PDGF-AA might have enhanced proteoglycan synthesis in chondrocytes and aid in cartilage healing. | (Wang and Xu, 2021) | |
| PDGF-BB might have prevented chondrocyte apoptosis based on the p38/Bax/caspase-3 pathway. | (Andia and Maffulli, 2018) | ||
| PDGF-BB might have boosted the proliferation of chondrocytes and the production of cartilage matrix. | (Belk et al., 2021) | ||
| EGF and TGF-α | The anabolic function of articular cartilage was enhanced by stimulating the EGFR signaling pathway. | (Singh and Harris, 2005) | |
| OA was more severe in EGFR-deficient mice than in wild-type mice. | (Rayego Mateos et al., 2018) | ||
| Lowering EGFR activity caused articular cartilage to be structurally, functionally, and mechanically weakened. | (Pest et al., 2014) | ||
| The levels of HBEGF and TGF-α increased during the development of cell clusters post-cartilage damage. | (Long et al., 2015) | ||
| IGF-1 | IGF might have increased the production of proteoglycan and collagen II while decreasing the production of MMP-13 within rat endplate chondrocytes. | (Shepard et al., 2013) | |
| IGF-1 reduced the production of reactive oxygen species, leading to antiapoptotic effects. | (Wei et al., 2021) | ||
| Detrimental | VEGF | Blocking the effects of VEGF and its receptor in animal OA models might have slowed the disease's progression. | (Haywood et al., 2003) |
| PRP with reduced VEGF levels enhanced OA healing. | (Giatromanolaki et al., 2001) | ||
| TNF-α | TNF-α might have boosted the synthesis of inflammatory cytokines and suppressed the production of proteoglycan and collagen II in chondrocytes, resulting in cartilage degeneration. | (Wang and He, 2018) | |
| Activated the NF-κB pathway promoting cartilage degradation. | (Murakami et al., 2000; Séguin and Bernier, 2003) | ||
| TNF-α might have lowered SOX-9 transcription factor expression and impaired respiratory chain efficiency. | (Jiranek et al., 1993; Merkel et al., 1999) | ||
| Ang-1 | Ang-1 stabilizes newly formed blood vessels by enlisting adjacent mesenchymal cells during VEGF-induced vessel formation. | (Park et al., 2005) | |
| SDF-1α | The SDF-1/CXCR4 signaling pathway might have caused a disruption in the equilibrium of aggrecan within the cartilage of rats suffering from osteoarthritis. | (Zheng et al., 2017) | |
| The level of SDF-1 in the subchondral bone rose in OA, causing subchondral osteosclerosis to form. | (Kanbe et al., 2002) | ||
| The SDF-1/CXCR4 pathway might have reduced irregular bone growth and blood vessel formation. | (Lu et al., 2019) | ||
| Unknown | CTGF | The expression of CTGF was upregulated in OA cartilage, promoting inflammation and cartilage damage through NF-κB. | (Blaney Davidson et al., 2006; Omoto et al., 2004) |
| It interacted with TGF-β and VEGF signaling pathways, suggesting a potential dual role in OA. | (Bazzazi et al., 2018; Lu et al., 2011) | ||
| FGF | FGFR1 signaling promoted matrix degradation; FGFR3 signaling promoted anabolic activity. | (Im et al., 2007; Weng et al., 2012) | |
| Contradictory roles occurred due to different receptor activation; the overall effect on OA remained unclear. | (Xie et al., 2020a; Xie et al., 2020b) | ||
| HGF | HGF, released by different cells, was increased in osteoarthritis and aided in tissue repair and remodeling. | (Mabey et al., 2014; Nagashima et al., 2001) | |
| Overexpression activated the c-Met pathway, inducing extracellular matrix degradation. | (Que et al., 2022) |
In exploring the potential effects of PRP on improving bone marrow mesenchymal-stromal-cells (BMSCs)’ regenerative capability, Ragni et al. (Ragni et al., 2022) identified 105 soluble factors and 184 EV-miRNAs in the secretome of PRP-treated BMSCs, respectively. Among them, several EV-miRNAs exhibited an immunomodulating capability at both the cell and the single-factor level and the ability to target enzymes that degrade the ECM characteristic of OA and pathways contributing to cartilage destruction. The data demonstrated that BMSCs incubation with PRP released therapeutic agents with strong anti-inflammatory capacity and provided a molecular basis for innovative therapies for OA treatment. Arafat and Kamel found that PRP combination therapy may have better outcomes than PRP alone (Zhang et al., 2022a). In a meta-analysis of clinical practice, the controlled clinical trial compared the efficacy of PRP with that of corticosteroids, HA, or placebo in treating knee OA (KOA) at 3, 6, and 12 months after administration, respectively. The data supported that PRP had the best overall results (Rodríguez-Merchán, 2022). Not coincidentally, a follow-up trial of 959 patients with KOA was mentioned in a literature review by Li et al. At 1, 2, 3, 6, and 12 months after PRP injections, total knee scores of patients were recorded, respectively. The group treated with PRP improved significantly than the baseline (Li et al., 2022). This study further proves that PRP helps promote tissue repair and reduce inflammation.
Although PRP has shown considerable effectiveness in the clinical management of OA, the enduring efficacy and safety of PRP warrant further study; in addition, PRP and its derivatives may affect the catabolism and inflammatory process of KOA, and the acceptability of different concentrations of PRP to individuals and the side effects of PRP are still urgent issues. More and more studies have chosen the DDSs, especially the PRP-EVs, to deliver PRP for the treatment of OA, and some animal experiments have been conducted. For example, Guo et al. observed the skin healing process of chronic wounds subjected to therapy with PRP-Exosomes in a diabetic rat model. Importantly, this study pioneered the demonstration of the feasibility of using exosomes to perform the function of PRP (Guo et al., 2017; Tao et al., 2017). The therapeutic roles of PRP-Exos in OA are mainly reflected in the promotion of chondrocyte migration and proliferation, the reduction of chondrocyte apoptosis and hypertrophy, and the inhibition of the release of inflammatory factors (Zhang et al., 2022a). Moreover, cross-species communication based on EVs has been reported multiple times. For instance, EVs derived from the human liver stellate cell line LX2 have been used in rat models (Li et al., 2017), and EVs derived from human MSCs have been applied in mouse and rat models (Monsel et al., 2015; Zhang et al., 2015). This species-specific PRP-Exos undoubtedly offers a promising research direction for the treatment of OA, especially considering the demonstrated potential of EVs in cross-species therapeutic applications. Liu et al. (Liu et al., 2019) established an IL-1β-provoked OA model and determined whether activated PRP (PRP—As) and PRP-Exos exhibited analogous biological effects in the management of OA. In in vitro experiments, they used primary rabbit chondrocytes to establish an OA model. They evaluated the therapeutic effects of PRP-As and PRP-Exos on OA through proliferation, migration and apoptosis assays. Results showed that PRP-Exos were statistically superior to PRP—As. Additionally, they aimed to confirm further that PRP-Exos alleviates OA through the Wnt/β-catenin cascade reactions. A KOA model was established to compare the therapeutic effects of PRP-Exos and PRP-As in vivo. The findings indicated that both PRP-Exos and PRP-As exhibited therapeutic effects on OA; however, the efficacy of PRP-Exos surpassed that of PRP—As, as evidenced by chondrocyte counts and the Osteoarthritis Research Society International (OARSI) scoring system. In summary, PRP-Exos acted as a carrier and contained PRP-derived GFs in this study, providing a new therapeutic approach to OA by triggering the Wnt/β-catenin cascade reactions. PRP-EVs not only attenuate OA by reducing the expression of TNF-α but also reverse the OA-induced down-regulation in type II collagen level and up-regulation of Wnt5a protein.
In fact, in addition to the study of mouse/rabbit OA models, dogs (Estes et al., 2021; Meeson et al., 2019), sheeps (Lovati et al., 2020), horses (Kupratis et al., 2022; van Loon and Macri, 2021) and other animals have also been used for the development of OA animal model. In contrast, the joint anatomy, cartilage morphology, and biomechanical function of these large animals are similar to those of human joints, providing more meaningful data for further clinical research. However, due to the complexity and heterogeneity of OA, the animal models currently used to replicate OA cannot fully reproduce the symptoms and pathological characteristics of human OA. This limitation has led to a lack of translational effects in most studies on OA treatment and the clinical outcomes of drugs in human subjects. Compared with in vivo models, in vitro engineered OA models are gaining increasing attention as a new frontier in OA research, including application such as tissue culture (Geurts et al., 2018), multicellular/tissue co-culture (Louer et al., 2012), 3D cell culture (Makarczyk et al., 2021), microtissue construction (Brandenberg et al., 2020), microphysiological systems, and organoids (Brandenberg et al., 2020; Hofer and Lutolf, 2021; Molinet et al., 2020; Schutgens and Clevers, 2020). For instance, Peng et al. (Peng et al., 2023) recently evaluated the therapeutic potential of human PRP and hyaluronic acid (hPRP/HA) in treating TMJ-OA from laboratory to human treatment. They investigated the effects and underlying mechanisms of hPRP/HA therapy for TMJ-OA in animal models, as well as conducted in vitro experiments (including 3D models) and clinical trials in patients with TMJ-OA. This study provides comprehensive evidence for the application of hPRP/HA in TMJ-OA treatment, spanning from the laboratory to the clinic and from animals to humans. Even though in vitro engineered OA models face many challenges and are expected to become a crucial model for clinical translation, they are merely prototypes that cannot replicate the entire structure of human bones and joints or create a physiological environment consistent with human OA (Dou et al., 2023). Recently, the integration of technologies such as genome editing, single-cell RNA sequencing, and lineage tracing has advanced the development and improvement of engineered OA models (Tong et al., 2022). It is anticipated that in the near future, a robust, physiologically relevant, and stable OA model will fundamentally transform drug development and testing. Such a model will provide more reliable and stable preclinical data for clinical trials of OA treatments.
To overcome the low retention rate and short therapeutic duration of PRP-Exos, Zhang et al. incorporated PRP-Exos into a thermosensitive hydrogel (Gel), aiming to increase the retention of PRP-Exos in joints. The results showed that this strategy enhanced cartilage protection after joint instability (Zhang et al., 2022a). Mata et al. (Mata et al., 2024) prepared gelatin/PRP microdroplets using an emulsion method to study the effects of platelet-derived GFs on the differentiation of MSCs into hyaline cartilage cells in a 3D environment. Subsequently, bone marrow MSCs were cultured in a microgel containing cartilage cells and combined with gelatin/PRP. In this study, gelatin microspheres effectively encapsulated PRP, significantly promoting the secretion and release of cartilage differentiation-promoting factors. Simultaneously, the microgel provided a 3D microenvironment, offering a surface for cell adhesion and the possibility of 3D cell-to-cell interactions. In summary, PRP delivery strategies for OA treatment offer advantages such as controlled drug release kinetics and tissue-specific targeting. It is foreseeable that drug delivery strategies will have more research and application in treating OA shortly.
5.1.2. Rheumatoid arthritis
Rheumatoid arthritis (RA) is a refractory autoimmune disease of synovial joints (Perera et al., 2024; van Delft and Huizinga, 2020). If left untreated, RA would lead to progressive corrosive joint damage, excessive comorbidities, and increased mortality (Myasoedova, 2021). RA is marked by infiltration of immune cells in the joints, which may cause inflammation, pain, edema, and joint degeneration. Up to now, some cascade signals in RA pathogenesis have been clarified, such as the T cell receptor (TCR) signal path and the CD40 signal path (Hasanov et al., 2018; Yanxia et al., 2017). Since the human leukocyte antigen DRB1 (Part of the primary histocompatibility complex class II receptor) is expressed in antigen-presenting cells (APCs), the interaction between APCs and CD4+ T cells via DRB1 could play a central role in RA pathogenesis (Viatte and Barton, 2017). The pathological changes of RA demonstrate the two sides of the autoantibody presence, which can either exacerbate the disease by promoting an inflammatory response and destroy tissue structure or help the diagnosis and treatment by acting as an indicator of disease severity or a potential therapeutic target, such as the expression of anti-citrullinated protein antibodies and rheumatoid factor (Rantapää Dahlqvist et al., 2003).
Local clinical manifestations of RA include joint deformities (gooseneck deformity, knob deformity, etc.), rheumatoid nodules, and rheumatoid vasculitis. In contrast, systemic clinical manifestations include symmetric polyarticular swelling and pain, morning stiffness, and weight loss. Its treatment principles include early treatment, the combination of drugs, and individualized treatment. Commonly used clinical drugs include NSAIDs, anti-rheumatic drugs (DMARDs), glucocorticosteroids, and so on (Lin et al., 2020). Despite significant advances in the treatment of RA, approximately 40 % of RA patients do not respond to drugs, and up to 20 % do not respond to any of the available medications (Perera et al., 2024). Additionally, the conventional treatments still have other shortcomings. For example, although methotrexate (MTX) is an excellent drug in the management of RA, it does not accumulate well at the site of inflammation. Long-term exposure to high doses of MTX can lead to serious side effects, whereas their concentration at the site of inflammation can not be controlled (Wang et al., 2022). Therefore, novel therapeutic approaches to reduce inflammation and protect bone tissue by modulating and inhibiting inflammatory mediators should be further explored.
The data have supported the idea that PRP actively removes inflammatory factors and promotes cartilage matrix recovery. Although the effects of PRP on OA have been recognized, RA is still significantly different in terms of pathological mechanisms from OA. In existing studies, it has been continuously verified that PRP can be used to manage RA disease. Saif et al. (Dalia et al., 2021) compared the outcomes between patients with monthly intra-articular steroids and those with PRP injections 3 times per month by the controlled variable method. The results showed that PRP was superior to steroids and had a more substantial inhibitory effect on the inflammatory cytokines IL-1β and TNF-α, which improved local joint inflammation, quality of life, and disease activity. Amanda et al. (Amanda Bezerra et al., 2021) evaluated the effects of photobiomodulation (PBM) and PRP on the inflammatory and oxidative stress parameters of acute arthritis in Wistar rats. It was concluded that PBM, in combination with PRP, had better anti-inflammatory and joint protective effects. Tong et al. (Tong et al., 2017b) investigated that PRP was used to treat type II collagen-induced arthritis (CIA) in mice. The data exhibited that the signs of arthritis, concentrations of anti-collagen antibodies, were decreased, accelerating tissue repair. This study has demonstrated that PRP can alleviate arthritis and reduce humoral and cellular immune responses. In another study by his team, rheumatoid fibroblast-like synoviocyte MH7 A cells were treated with lipopolysaccharide (LPS) to mimic the RA condition. The results showed that PRP can inhibit synoviocyte fibroblasts and modulate PI3K/Akt signals (Tong et al., 2017a). From the experiments described above, PRP can adjunctively treat RA by down-regulating inflammatory cytokines and suppressing synovial fibroblasts (Dejnek et al., 2022).
Combining PRP with NPs may be a better choice for treating RA. Pan et al. (Pan et al., 2020) combined PRP-chitosan thermosensitive hydrogel containing black phosphorus nanosheets (BPNs) to treat RA (Fig. 7A). This thermosensitive gel guards articular cartilage by alleviating friction between other surrounding tissues. Edema in arthritic mice was significantly relieved by BPNS/chitosan/PRP thermosensitive gel. As shown in Fig. 7B, the authors first used CCK-8 to demonstrate the impact of various treatment cohorts on the survival rate of proliferating synovial cells. However, the results showed that the BP/chitosan/PRP group did not exhibit significant therapeutic advantages. Nevertheless, the authors believe that the mechanical properties of the hydrogels for MSC adhesion and proliferation and the biological characteristics of PRP are unique. Further, combining Annexin V-FITC/PI fluorescence dyeing, the authors revealed that after culturing mouse OA synovial cells within BP/chitosan/PRP thermosensitive hydrogel, the apoptosis and cellular senescence rates significantly increased. In vivo experiments, the authors combined histopathological analysis to evaluate the therapeutic effects of BP/chitosan/PRP thermosensitive hydrogel. The clinical index of CIA mice is shown in Fig. 7C. Both in vitro and in vivo results of this study suggested that the combination of NPs and PRP potentiates the treatment of RA. Although nanoparticle technology, in combination with PRP, is also expected to provide a new strategy for treating RA, more clinical trials are needed to support PRP's clinical outcomes in treating RA.
Fig. 7.
The roles of BPNs/Chitosan/PRP thermosensitive hydrogel for treating RA. (A) The scheme of black phosphorus nanosheets (BPNs) contains PRP-chitosan thermosensitive injectable gel for phototherapy and biotherapy treatment of RA. (B) The CCK-8 assay proved the treatment of BPNs/Chitosan/PRP thermosensitive hydrogel on the hyperplastic synovial cells. (C) The OA index of BPNs/Chitosan/PRP thermosensitive gel without or with near-infrared (NIR) (Pan et al., 2020). Copyright © 2020, The Wenzhen Pana (s).
5.2. Fractures
A fracture is a break in the integrity and continuity of the bone due to trauma or skeletal disease. Clinical manifestations of fractures mainly include pain and tenderness, swelling and ecchymosis, and dysfunction (Volodymyr et al., 2015). For the treatment of fractures, bone healing is a critical and complicated process in orthopedics, which usually undergoes three phases, including hematoma inflammatory agitation, primitive bone scab formation, and bone scab remodeling and shaping. Under normal healing conditions, bone can fully regenerate. Delayed healing or bone nonunion is a common problem in the clinical treatment of fractures, possibly leading to disability (Chen et al., 2020; Van Lieshout and Den Hartog, 2021). Traditional treatments such as bone grafts and bone substitution materials take the risk of rejection and infection (Amit and Eshita, 2020). The treatment of massive fractures remains a persistent challenge for clinical treatment. Regenerative medicine has developed some innovative therapy approaches for the treatment of fractures, in contrast to other regenerative approaches, such as bone morphogenetic proteins (MBP) or stem cell therapy.
Unlike the other methods, PRP therapy delivers a unique mixture containing autologous cytokines and GFs that may reduce the risk of immune rejection related to synthetic or allogeneic materials. In treating fracture nonunion, PRP is effective in accelerating the healing of bone defects, new bone formation and improving the quality of new bone (Blake et al., 2024). Compared to conventional treatments, PRP has been shown to have a reliable efficacy for shortening bone healing time. In an experiment evaluating platelet-rich growth factor (PRGF) ‘s role in treating dog fractures, Sergio et al. observed faster fracture healing in the PRGF-treated group (López et al., 2019). In a pilot study investigating whether PRP could reduce pain and improve movement function in distal radius fractures, Namazi et al. (Namazi and Mehbudi, 2016) confirmed that PRP had a significant prognostic effect on patients with intra-articular distal radius fractures, reducing pain during activity and promoting recovery of distal radius function. Although PRP has shown positive effects in the treatment of fractures, the premature dissolution of PRP often leads to the exhaustion of cytokines, and this dissolution may even occur before bone formation (Griffin et al., 2009; Plachokova et al., 2008). Therefore, to enhance the beneficial effect of PRP, it is necessary to delay the release of PRP cytokines through specific strategies. In recent years, some nanoscale DDSs have been designed for particular targeting of bone cells due to their advanced features, including the enhancement of drug solubility, the improvement of drug stability, and inhibition of drug degradation, which allows the drug to reach its target site and accurately accumulate at the fracture site. The advanced features of DDSs also include high local drug utilization and efficacy, reduced adverse effects associated with off-target delivery, and rapid removal from the body circulation (Chen et al., 2020).
Some of the necessary targeted DDSs for fracture healing are shown below. First, hydrogels are biocompatible and adjustable in shape and size to suit different forms of bone defects. The gels allow the controlled release of therapeutic reagents and delivery of therapeutic agents to specific cell types, and they can be used in bone re-formation (Li et al., 2021). For example, Gao et al. (Gao et al., 2019) prepared a novel composite hydrogel using PRP and sodium alginate (SA) to support the proliferation of bone marrow-derived MSCs and their chondrogenesis. Surprisingly, during the experiment, it was found that PRP significantly reduced the gelation time and swelling ratio of the alginate hydrogel, resulting in a more uniform gel structure with excellent biocompatibility. Additionally, compared to pure SA hydrogels, the compressive mechanical strengths of the SA/PRP composite hydrogel were enhanced. Studies showed that fibrin hydrogels based on patient PRP can be used for tissue regeneration by loading highly biocompatible materials. Additionally, when the platelets are activated by calcium and thrombin, they release many GFs contained in PRP, stimulating angiogenesis and cell mitotic and metabolic activities. (Zhang et al., 2013) Based on the research above, Nedorubova et al. (Nedorubova et al., 2022) developed polylactic acid (PLA) particles impregnated with BMP2 polymers and encapsulated in PRP-contained chitosan hydrogels or fibrin hydrogels. Experimental results showed that the PLA/PRP group exhibited the most significant effects on osteoinduction. Therefore, it was demonstrated that the developed drug delivery system is a safe and effective bone material. This PRP-based fibrin hydrogel holds promise for applications in bone restoration owing to its capability to support osteoinduction and biocompatibility. Fernandes et al. formulated alginate hydrogel microspheres infused with PRP, which facilitated the regulated release of PRP and promoted the osteogenic differentiation of BMP-2-modified MSCs. The in vitro and in vivo data showed that the controlled release of PRP in alginate beads could accelerate bone healing (Fernandes et al., 2016). This study indicates that PRP and MSCs co-blended with injectable composites can promote bone regeneration in fracture models.
5.3. Soft tissue injury and inflammation
5.3.1. Articular cartilage injury
Articular cartilage is a hyaline cartilage lining the surface of adult joints (Pei et al., 2022); it consists mainly of chondrocytes and ECM. The cartilage is typically categorized into four distinct layers: tangential (superficial), transitional (middle), radial (deep), and calcified areas (Markhardt et al., 2022). Although chondrocytes make up only 2 % of the total cartilage volume, they primarily perform cartilage functions. Chondrocytes maintain the biomechanical environment of cartilage that plays a role in weight bearing, shock absorption, and protection of the articular surfaces. Since cartilage has no connective tissue, such as blood vessels, nerves, or lymph, cartilage cannot obtain nutrients and eliminate wastes directly through these tissues. It mainly takes up nutrients from surrounding tissues in a diffusion way. Therefore, when the neighboring tissues of the cartilage are damaged, the supply of nutrients to the cartilage may be affected, leading to degeneration and deterioration of the cartilage (Pei et al., 2022). When articular cartilage is damaged, the chondrocytes become edematous and necrotic. Once the ECM is destroyed, the cartilage's original smooth surface and cushioning function is impaired, often accompanied by pain, swelling dysfunction, and other severe discomfort (Philipp et al., 2022). Existing treatments include medication, surgery, cell therapy, cartilage tissue engineering techniques, arthroscopic free bone removal, etc. However, these approaches can only alleviate pain and postpone degeneration, not fundamentally repair cartilage. Novel therapeutic strategies include stem cell transplantation and GF therapy. These methods have repaired cartilage injury to some extent. Stem cells can directly inhibit synovial activation and indirectly improve cartilage damage by creating a tissue-repairing microenvironment to stimulate tissue repair by recruiting local endogenous stem cells (Schmitz et al., 2022). Tucker et al. (Tucker et al., 2021) conducted randomized, double-blind clinical trials of 18 sufferers to explore the effects of PRP on MSCs gene expression and synovial protein levels and found a significant increase in A2M (α-2-macroglobulin) protease after PRP treatment (P = 005). PRP was hypothesized to modulate the local knee synovial microenvironment by regulating matrix degradation, inflammatory milieu, and vascular GFs to promote articular cartilage recovery. However, the novel therapeutic approach also faces many problems and challenges. They include the non-unified standardization of PRP preparation and the lack of standard guidelines for PRP treatment at the clinic stage. Moreover, the promotion of biological healing through prolonged and sustained release of the active ingredients in PRP still needs to be explored through extensive experiments.
Bansal et al. (Bansal et al., 2021) found that the symptomatic betterment of patients in the PRP-administered group could last 12 months. Thus, PRP plays a critical role in the enduring restoration of cartilage defects. Fortier et al. (Fortier et al., 2016) demonstrated that PRP combined with Biocartilage (e.g., isogenic articular cartilage particles) could more significantly improve cartilage repair than microfracture alone in an equine model (Fortier et al., 2016). Lourdes et al. (Alcaide Ruggiero et al., 2023) evaluated the chondrogenic regenerative potential of a self-synthesized matrix created from particulate cartilage and PRP implantation in treating full-thickness chondral defects of sheep. The data showed that the quality of chondrocytes in the injured animals was improved in the group using the combination method and that the phenotype of the chondrocytes was very similar to that of healthy cartilage. Not only that, research has found that microRNAs (miRs) in EVs released by MSCs have the potential to promote cartilage regeneration and impede the progression of the disease. Exos are a promising therapeutic carrier for the future. However, further validation of the safety and efficacy of Exos is also needed (Yang et al., 2024).
5.3.2. Bursitis
A bursa is a sac-like fibrous connective tissue structure within which synovial fluid can reduce friction between joints and soft tissues. Bursitis is a common joint disease that primarily influences the synovium (the lining inside the joint), causing inflammation and pain. Bursitis usually occurs in the shoulder, elbow, and hip due to long-term wear and tear or overuse (Kwiatkowski and Płomiński, 2004). Common bursitis includes prepatellar bursitis, hawksbill bursitis, greater trochanteric bursitis, and posterior heel bursitis. Some bursitis may be treated conservatively with adductor muscle stretching and strengthening (Garrasi et al., 2023). However, recalcitrant bursitis, such as refractory rotor bursitis, may require surgical treatment (Aaron et al., 2011). Additionally, physical therapy includes heat, cold, electrotherapy, ultrasound, and medications such as oral antibiotics, NSAIDs, local injections of corticosteroids, and PRP, which are also used for treating bursitis.
A flurry of experiments has demonstrated PRP's feasibility for treating bursitis. Autologous PRP repairs the damaged synovial membranes and soft tissues, reduces inflammation, and promotes tissue regeneration. PRP effectively relieves the symptoms of bursitis and improves joint function. Jessica et al. (Gilbertie et al., 2022) developed a biologic formulation of PRP called BIO-PLY (biologically active components enriched in platelet plasma lysate), which has the potential in vitro anti-bacterial capability against free-floating biofilm aggregates of S. aureus within the synovial fluid (Fig. 8A). The in vitro data from horse chondrocytes and synoviocytes have shown that BIO-PLY can protect these joint cells from inflammation. The efficacy of BIO-PLY in vivo from a horse model with symptoms of infectious arthritis revealed that animals administered with the antibiotic amikacin (AMK) in combination with BIO-PLY had fewer bacteria in both synovial tissues as well as synovial fluid and better local and systemic inflammation scores (Fig. 8B-C). Most meaningfully, the AMK + BIO-PLY combination inhibited the loss of infection-related chondroproteoglycan amount in articular cartilage and reduced synovial tissue inflammation and fibrosis (Fig. 8D-E). The results demonstrated the AMK + BIO-PLY as a novel clinical choice to enhance antimicrobial features against the bacteria on the synovial biofilms. Araya et al. (Araya et al., 2020) established a model of monosodium iodoacetate (MIA)-induced arthritis. They confirmed that pure PRP injections were the most effective treatment for reducing pain-related behaviors and inhibiting synovial inflammation and sensitization. Carl et al. recruited 24 elderly individuals with mild or moderate KOA combined with suprapatellar bursitis to evaluate the effectiveness of PRP. The data suggested that at least two PRP injections per month may be beneficial in treating patients with mild to moderate KOA combined with suprapatellar bursitis. In addition, small EVs from human OA chondrocytes had high concentrations of Cx43. They induced an aged phenotype of synovium cells, targeted chondrocytes, and osteoblasts, leading to inflammation and abnormal joints. The secretion of senescence-related secretion-related phenotypic proteins such as IL-1β, MMP, and IL-6 was beneficial to the formation of microenvironments. Cx43 on the EV surface may be a new diagnostic marker to evaluate the disease progression and a novel target to cure OA (Varela Eirín et al., 2022). Direct studies of PRP through drug delivery systems for better action in treating bursitis are less at this stage. This is an area to be further explored.
Fig. 8.
(A) Diagram of the experimental design to test the protective efficacy of BIO-PLY to equine synoviocytes and chondrocyte cells of the joint. The efficacy of BIO-PLY in vivo (B) Results of H&E, SafO stained cartilage and (C) Overall total OARSI scores showed that scores for numbers of chondrocyte clones and focal cell loss were reduced in AMK + BIO-PLY administered animals when compared with AMK alone. (D) Representative SafO-stained osteochondral sections and (E) Morphometric analysis of SafO staining percentage of the total surface area of articular cartilage revealed a reduced SafO staining percentage in the non-mineralized cartilage layers of AMK-administered joints when compared with AMK + BIO-PLY-administered joints (Gilbertie et al., 2022). Copyright © 2022, The Jessica M. Gilbertie (s).
5.3.3. Achilles tendonitis
Degenerative changes of the Achilles tendon, including increased vascularization and inflammatory response, usually characterize Achilles tendinopathy (AT). Clinical symptoms include swelling, pain, torticollis, localized tenderness, fever, and dysfunction. MRI and ultrasonography can diagnose AT (Longo et al., 2018). Treatment of AT usually requires long-term and invasive therapies, including therapeutic endoscopy or surgery. To halt the progression of chronic AT, it is essential to reduce excessive inflammation at an early stage. NSAIDs or corticosteroids are commonly prescribed to manage inflammation. However, the high doses and long-term treatment can lead to serious side effects (Kim et al., 2022). Continuous efforts have been made to find a practical approach for AT injuries to enable patients to return to sports more quickly. Several new methods have been introduced for tissue-engineered bioenhancement of tendons, such as gene therapy, GFs, and MSCs. Growing evidence shows that some GFs can inhibit inflammation reactions, accelerate extracellular matrix production, and promote AT repair (Lin et al., 2023). PRP-containing GFs could represent an alternative treatment modality to reduce the discomfort of AT and encourage functional recovery of the AT (Desouza et al., 2023).
The mechanisms of PRP treatment of AT are: i) release some GFs to promote tissue repair and regeneration; ii) reduce pain and inflammation; iii) improve blood supply to accelerate the functional recovery of the Achilles tendon; and iv) promote the activation of fibroblasts to accelerate tissue repair. Park et al. (Park et al., 2024) used rabbits as model animals to evaluate the impacts of PRP on peritendinous fascia healing of AT after allograft transplantation. The allograft transplantation in the PRP-treated group exhibited greater tensile strength, higher macroscopic assessment scores, and an accelerated histological healing process compared to the control group. It suggests that the PRP accelerates the healing process for ruptured Achilles tendon ruptures via allograft tendon transplantation. Alsousou et al. (Alsousou et al., 2015) evaluated the impacts of PRP on the healing tissue of acute AT ruptures. Topical application of PRP promotes collagen I deposition, cellular reduction, and an increase of glycosaminoglycan content, thereby increasing the healing maturity of tendon tissue. More studies are essential to elucidate the chronic effects of PRP administration in musculoskeletal disorders. Monto et al. found that PRP could effectively treat chronic and persistent AT (Monto, 2012). Xu et al. (Xu et al., 2017) found that PRGF (activated PRP) significantly enhanced cellular DNA synthesis, increased viability, and promoted the proliferation, migration and recruitment of tendon-derived stem cells (TDSCs). PRP is an activator of TDSCs and improves the quality of early healing of Achilles tendon rupture. Guelfi et al. (Guelfi et al., 2015) carried out a retrospective study to assess the clinical benefits of patients administered single PRP injections. The follow-up results have demonstrated that a single administration of PRP is an attractive and safe choice for non-insertional chronic recalcitrant mid-portion AT. However, Kearney et al. (Kearney et al., 2021) compared the effects of a single injection of PRP with a sham injection (subcutaneous dry needle insertion) on mid-segment AT disease in adult patients. The data concluded that a single injection of PRP did not shorten the healing time of chronic mid-segment AT compared to sham injection. This result led to a controversy regarding PRP in treating AT. In the follow-up discussion of this trial, it was suggested that factors such as the imprecise injection of PRP into the tendinopathic area of the tendon and the uncontrolled quality of the injected PRP samples may have compromised the trial results. This indicates that these issues could have specifically affected the reliability and validity of the findings.
For the problem of non-precision injection, some studies have begun to use nanocarriers to achieve precision drug delivery. Li et al. printed hydrogel particles loading PRP to alleviate chronic inflammation due to AT injury. Hopefully, tendon-derived stem cells could maintain homeostasis and repair injury. PRP-loaded gelatine methacryloyl microparticles printed by 3D printing could affiliate stem cells' differentiation into tendon tissue cells and inhibit the inflammation syndrome by suppressing the PI3K-AKT signaling regulation. The in vivo results supported that the defected tendons were structurally and functionally repaired (Li et al., 2023).
6. Conclusions and perspectives
PRP is from autologous blood and is a concentrated suspension of platelet-rich blood obtained from the patient's blood. It represents a novel therapeutic tool in the treatment of orthopedic diseases. Platelets can produce GFs such as IGF-1, PDGF, EGF-HGF, TGF-β, bFGF, and VEGF, etc., which could regulate wound healing. It can accelerate tissue repair and regeneration (Cecerska Heryć et al., 2022). Compared to traditional surgical treatments, PRP therapy is less invasive, reduces the risk of infection, and mitigates rejection. PRP is becoming a non-surgical option for degenerative musculoskeletal diseases because of its potential to reverse joint space thinning, cartilage damage, and associated pain (Pirri et al., 2024). Some studies have also confirmed that PRP significantly alleviates pain at the injury site and improves joint function. In sports medicine, PRP treats sports-related soft tissue injuries such as tendons, ligaments, and muscle injuries (Middleton et al., 2012). In particular, PRP injections have been associated with chronic pain relief and functional improvement in the setting of ulnar collateral ligament pathology of the elbow, including lateral tennis elbow (Anthony et al., 2022). However, neither the clinical efficacy nor the PRP action mechanism has been thoroughly characterized. It has also been noted that the effectiveness of PRP may change with the types of injury and timing of treatment. Particularly, due to individual differences and various formulations, the efficacy of PRP is still worth further discussion, leading to diverse therapeutic effects. In different studies, multiple formulations of PRP may elucidate the disparate effects observed in treating diseases. Future studies should optimize the therapeutic concentration, route of administration, and timing of PRP further to achieve the desired effects in specific tissues. As described previously, the treatment of PRP requires the patient's permission to draw blood for centrifugal collection of platelets, which requires strict sterile conditions and procedures. However, there is still no unified standard of medical institution qualification requirements for the preparation and clinical application of PRP. In addition, due to the differences in quality and source of platelets and the differences in preparation materials, methods, personnel, and technology, the PRP prepared must have specific variations. This also makes it difficult to manage or further develop PRP into a commercial drug. It is suggested that effective PRP preparation methods (double centrifugation or single centrifugation) and administration (single or multiple injections) are needed to study and standardize for further therapeutic development systematically (Andersen et al., 2021). Many high-quality experimental studies still need to verify the duration of PRP effects and the onset of action after use. Most articles state that the effects of PRP are usually time-limited and not long-lasting (Seckin et al., 2022). Regarding the controversy over the effectiveness and durability of PRP, more discussion is needed on the molecular mechanisms related to PRP. We must acknowledge that not every cytokine PRP produces is advantageous; some may cause uncertain or adverse effects. In response to this issue, current research is beginning to explore the use of high-concentration PRP GFs and cytokines levels as commercial alternatives to PRP, which might bring new opportunities for its clinical application. In addition, PRP's direct or indirect transportation into bone tissue has to face problems such as uncontrolled release, low targeting, weak accessibility, and short half-life time. With the help of microencapsulation or nanotechnology, the shortcomings of traditional drug delivery methods can be significantly improved to enhance drug bioavailability, increase the drug release rate, reduce adverse reactions, etc., so that PRP can play a better role in orthopedics.
Although DDS, such as microspheres and hydrogels, can improve treatment outcomes to some extent (Oh et al., 2018). However, the accumulation and distribution of these passive targeting DDS in the body are limited due to natural fluid circulation and diffusion concentration gradients. Moreover, the highly variable drug loading rates among DDS contribute to the differences in therapeutic effects. Recent studies have shown that technologies like implantable systems (Battiston et al., 2021; Freedman et al., 2022), microneedles (Than et al., 2018; Yu et al., 2018), microchips (Shin et al., 2021), and micro-robots (Sridhar et al., 2022) are being increasingly used to construct active targeting DDS. These technologies overcome the limitations of traditional passive DDS. Notably, micro-robots have gained significant attention due to their exceptional controllability. These micro-robots can be programmed to have a scalable size range from millimeters to micrometers, tunable payload capacities, and enhanced drug protection with minimal leakage (Song et al., 2022). More importantly, they can achieve precise navigation in complex fluid environments and penetrate hard-to-reach tissues (Wu et al., 2019). The emergence of these micro-robots undoubtedly provides more stable and reliable preclinical research support for the clinical translation of PRP based on DDS.
While PRP delivery with MPs or NPs has excellent therapeutic potential, the field still needs new in vivo models combined with robust imaging methods. Compared to MPs, NPs have greater advantages. However, the clinical translation of NPs poses greater challenges than that of MPs. Three essential factors must be considered for the clinical translation of NPs: toxicity, distribution, and ultimate fate of NPs in vivo. Although NPs used for drug delivery must be constructed from FDA-approved materials, their interactions with blood components remain to be determined. Studies have shown that different NPs may promote platelet aggregation and/or activation, or exert anti-platelet effects (Jun et al., 2011; Stevens et al., 2009). If the interaction between these NPs and platelets is not adequately addressed, it could further contribute to the development of cardiovascular diseases. Therefore, when researching the use of NPs to deliver PRP, the biological and toxicological characteristics of the nanomaterials should be prioritized at first. Second, it is critical to track the position of NPs in vivo for safety reasons. Advanced imaging techniques, such as PET, MRI, and fluorescence imaging, can be used for real-time in vivo tracking of NPs in joints or bone. Third, scientists should conduct further studies to fully understand the fate of NPs in the joint space and explore the biocompatibility, degradation rate, and immune response of NPs in vivo. Second, the interaction of NPs with synovial fluid remains unclear. The synovial fluid is the first substance with which the NPs contact after injection in the joint, thus significantly influencing the properties and functions of the NPs in the joint cavity. In addition, the joint's interaction mechanisms between NPs and different cell types (e.g., immune cells and chondrocytes) must be explored further (Wen et al., 2023). In the future, PRP is promising for treating orthopedic diseases through DDSs. However, how to deliver it safely, efficiently, and accurately still needs to be explored with many high-quality studies.
CRediT authorship contribution statement
Mingdong Liu: Writing – original draft, Visualization, Methodology, Formal analysis. Jiaxin Ding: Writing – original draft, Validation, Methodology, Data curation. Yanbo Peng: Software, Investigation. Jialin Fang: Writing – original draft, Visualization. Man Zhao: Writing – original draft, Software, Investigation. Wenyuan Zhang: Writing – original draft, Software, Investigation. Haotian Chen: Writing – original draft, Formal analysis. Jian Zhang: Writing – original draft, Validation. Haisheng Peng: Writing – original draft, Resources, Funding acquisition, Conceptualization. Qun Wang: Writing – review & editing, Supervision, Resources, Conceptualization.
Declaration of competing interest
None.
Acknowledgments
Dr. Peng thanks the support from the Joint fund of Shaoxing University and Shaoxing Institute of Zhejiang University (2023LHLG007), the Heilongjiang Provincial Natural Science Foundation (no. ZD2016013) and the National Natural Science Foundation of China (no. 81671814).
Contributor Information
Haisheng Peng, Email: fisher1688@163.com.
Qun Wang, Email: qunwang@iastate.edu.
Data availability
Data will be made available on request.
References
- Aaron D.L., Patel A., Kayiaros S., Calfee R. Four common types of bursitis: diagnosis and management. Am. Acad. Orthop. Surg. 2011;19:359–367. doi: 10.5435/00124635-201106000-00006. [DOI] [PubMed] [Google Scholar]
- Alcaide Ruggiero L., Molina Hernández V., Morgaz J., Fernández Sarmiento J.A., Granados M.M., Navarrete Calvo R., Pérez J., Quirós Carmona S., Carrillo J.M., Cugat R., Domínguez J.M. Particulate cartilage and platelet-rich plasma treatment for knee chondral defects in sheep. Knee Surg. Sports Traumatol. Arthrosc. 2023;31:2944–2955. doi: 10.1007/s00167-022-07295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsousou J., Thompson M., Harrison P., Willett K., Franklin S. Effect of platelet-rich plasma on healing tissues in acute ruptured Achilles tendon: a human immunohistochemistry study. Lancet. 2015;385:S19. doi: 10.1016/S0140-6736(15)60334-8. [DOI] [PubMed] [Google Scholar]
- Alves R., Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2017;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanda Bezerra G., Júlia Leme B., Bruna Silva G., Acácio A.P., Maíra F., Marcelo Augusto Marretto E., Gaspar de Jesus Lopes F., Fernando Russo Costa B. Photobiomodulation (λ=808nm) and platelet-rich plasma (PRP) for the treatment of acute rheumatoid arthritis in wistar rats. J. Lasers Med. Sci. 2021;12 doi: 10.34172/jlms.2021.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit L., Eshita S. Prospective aspects of regeneration in orthopaedics: a review. J. Pharm. Res. Int. 2020;32:116–125. [Google Scholar]
- Andersen C., Wragg N.M., Shariatzadeh M., Wilson S.L. The use of platelet-rich plasma (PRP) for the management of non-union fractures. Curr. Osteoporos. Rep. 2021;19:1–14. doi: 10.1007/s11914-020-00643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andia I., Maffulli N. A contemporary view of platelet-rich plasma therapies: moving toward refined clinical protocols and precise indications. Regen. Med. 2018;13:717–728. doi: 10.2217/rme-2018-0042. [DOI] [PubMed] [Google Scholar]
- Anitua E., Troya M., Falcon-Pérez J.M., López-Sarrio S., González E., Alkhraisat M.H. Advances in platelet rich plasma-derived extracellular vesicles for regenerative medicine: a systematic-narrative review. Int. J. Mol. Sci. 2023;24:13043. doi: 10.3390/ijms241713043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony G., Anak Agung Ngurah Ronny K., Saputra¬¬ I.K.A.K., Laksana I.K.M. Platelet-rich plasma (PRP) on orthopedic clinical daily practice: a literature review. Intisari Sains Med. 2022;13:341–346. [Google Scholar]
- Antich Rosselló M., Forteza Genestra M.A., Monjo M., Ramis J.M. Platelet-derived extracellular vesicles for regenerative medicine. Int. J. Mol. Sci. 2021;22:8580. doi: 10.3390/ijms22168580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya N., Miyatake K., Tsuji K., Katagiri H., Nakagawa Y., Hoshino T., Onuma H., An S., Nishio H., Saita Y., Sekiya I., Koga H. Intra-articular injection of pure platelet-rich plasma is the most effective treatment for joint pain by modulating synovial inflammation and calcitonin gene-related peptide expression in a rat arthritis model. Am. J. Sports Med. 2020;48:2004–2012. doi: 10.1177/0363546520924011. [DOI] [PubMed] [Google Scholar]
- Ayers L., Nieuwland R., Kohler M., Kraenkel N., Ferry B., Leeson P. Dynamic microvesicle release and clearance within the cardiovascular system: triggers and mechanisms. Clin. Sci. 2015;129:915–931. doi: 10.1042/CS20140623. [DOI] [PubMed] [Google Scholar]
- B Y., K C., J H., C M., G F., B Z., S Z. Effect of different doses of transforming growth factor-β₁ on cartilage and subchondral bone in osteoarthritic temporomandibular joints. Br. J. Oral Maxillofac. Surg. 2013;51:241–246. doi: 10.1016/j.bjoms.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Badade P.S., Mahale S.A., Panjwani A.A., Vaidya P.D., Warang A.D. Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J. Dent. Res. 2016;27:300–304. doi: 10.4103/0970-9290.186231. [DOI] [PubMed] [Google Scholar]
- Bakhaidar R., O'Neill S., Ramtoola Z. PLGA-PEG nanoparticles show minimal risks of interference with platelet function of human platelet-rich plasma. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21249716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A.C. van de Loo F.A. van Beuningen H.M. Sime P. van Lent P.L. van der Kraan P.M. Richards C.D. van den Berg W.B. 2001 Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation Osteoarthr. Cartil. 9 128 136. [DOI] [PubMed]
- Bansal H., Leon J., Pont J.L., Wilson D.A., Bansal A., Agarwal D., Preoteasa I. Author Correction: Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021;11:18612. doi: 10.1038/s41598-021-98365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barani M., Paknia F., Roostaee M., Kavyani B., Kalantar-Neyestanaki D., Ajalli N., Amirbeigi A. Niosome as an effective nanoscale solution for the treatment of microbial infections. Biomed. Res. Int. 2023;2023 doi: 10.1155/2023/9933283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiston K., Parrag I., Statham M., Louka D., Fischer H., Mackey G., Daley A., Gu F., Baldwin E., Yang B., Muirhead B., Hicks E.A., Sheardown H., Kalachev L., Crean C., Edelman J., Santerre J.P., Naimark W. Polymer-free corticosteroid dimer implants for controlled and sustained drug delivery. Nat. Commun. 2021;12:2875. doi: 10.1038/s41467-021-23232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzazi H., Zhang Y., Jafarnejad M., Isenberg J.S., Annex B.H., Popel A.S. Computer simulation of TSP1 inhibition of VEGF-Akt-eNOS: an angiogenesis triple threat. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzad H. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: part I. PubMed. 2011;2:205–212. [PMC free article] [PubMed] [Google Scholar]
- Belk J.W., Kraeutler M.J., Houck D.A., Goodrich J.A., Dragoo J.L., McCarty E.C. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am. J. Sports Med. 2021;49:249–260. doi: 10.1177/0363546520909397. [DOI] [PubMed] [Google Scholar]
- Bendinelli P., Matteucci E., Dogliotti G., Corsi M.M., Banfi G., Maroni P., Desiderio M.A. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J. Cell. Physiol. 2010;225:757–766. doi: 10.1002/jcp.22274. [DOI] [PubMed] [Google Scholar]
- Blake B., Smith R.P., Alec R., Augustus D.M., Ian D.H. Advances with platelet-rich plasma for bone healing. Biologics. 2024;2024:29–59. doi: 10.2147/BTT.S290341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson E.N., Vitters E.L., Mooren F.M., Oliver N., Berg W.B., van der Kraan P.M. Connective tissue growth factor/CCN2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage. Arthritis Rheum. 2006;54:1653–1661. doi: 10.1002/art.21795. [DOI] [PubMed] [Google Scholar]
- Bohren Y., Timbolschi D.I., Muller A., Barrot M., Yalcin I., Salvat E. Platelet-rich plasma and cytokines in neuropathic pain: a narrative review and a clinical perspective. Eur. J. Pain. 2022;26:43–60. doi: 10.1002/ejp.1846. [DOI] [PubMed] [Google Scholar]
- Brandenberg N., Hoehnel S., Kuttler F., Homicsko K., Ceroni C., Ringel T., Gjorevski N., Schwank G., Coukos G., Turcatti G., Lutolf M.P. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 2020;4:863–874. doi: 10.1038/s41551-020-0565-2. [DOI] [PubMed] [Google Scholar]
- BW O., JC P., R H.I.T.V., AJH V. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am. J. Sports Med. 2019;47:479–487. doi: 10.1177/0363546517746112. [DOI] [PubMed] [Google Scholar]
- Cai Y., Wang Z., Liao B., Sun Z., Zhu P. Anti-inflammatory and chondroprotective effects of platelet-derived growth factor-BB on osteoarthritis rat models. J. Gerontol. 2023;78:51–59. doi: 10.1093/gerona/glac118. [DOI] [PubMed] [Google Scholar]
- Cardoneanu A., Macovei L.A., Burlui A.M., Mihai I.R., Bratoiu I., Rezus I.I., Richter P., Tamba B.-I., Rezus E. Temporomandibular joint osteoarthritis: pathogenic mechanisms involving the cartilage and subchondral bone, and potential therapeutic strategies for joint regeneration. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo C., Roffi A., Grigolo B., Mariani E., Pratelli L., Merli G., Kon E., Marcacci M., Filardo G. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed. Res. Int. 2016;2016:6591717. doi: 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecerska Heryć E., Goszka M., Serwin N., Roszak M., Grygorcewicz B., Heryć R., Dołęgowska B. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84–94. doi: 10.1016/j.cytogfr.2021.11.003. [DOI] [PubMed] [Google Scholar]
- Cervelli V., Lucarini L., Spallone D., Palla L., Colicchia G.M., Gentile P., De Angelis B. Use of platelet-rich plasma and hyaluronic acid in the loss of substance with bone exposure. Adv. Skin Wound Care. 2011;24:176–181. doi: 10.1097/01.ASW.0000396302.05959.d3. [DOI] [PubMed] [Google Scholar]
- Chahla J., Cinque M.E., Piuzzi N.S., Mannava S., Geeslin A.G., Murray I.R., Dornan G.J., Muschler G.F., LaPrade R.F. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J. Bone Joint Surg. Am. 2017;99:1769–1779. doi: 10.2106/JBJS.16.01374. [DOI] [PubMed] [Google Scholar]
- Chen R.R., Mooney D.J. Polymeric growth factor delivery strategies for tissue engineering. Pharm. Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- Chen J., Ashames A., Buabeid M.A., Fahelelbom K.M., Ijaz M., Murtaza G. Nanocomposites drug delivery systems for the healing of bone fractures. Int. J. Pharm. 2020;585 doi: 10.1016/j.ijpharm.2020.119477. [DOI] [PubMed] [Google Scholar]
- Childs C., Proper J., Tucker R., Moses H. Serum contains a platelet-derived transforming growth factor. Proc. Natl. Acad. Sci. USA. 1982;79:5312–5316. doi: 10.1073/pnas.79.17.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.H., Blanco A., Stealey S., Duan X., Case N., Sell S.A., Rai M.F., Zustiak S.P. Micro-clotting of platelet-rich plasma upon loading in hydrogel microspheres leads to prolonged protein release and slower microsphere degradation. Polymers (Basel) 2020;12 doi: 10.3390/polym12081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueh K.S., Huang K.H., Lu J.H., Juan T.J., Chuang S.-M., Lin R.-J., Lee Y.-C., Long C.-Y., Shen M.-C., Sun T.-W., Juan Y.-S. Therapeutic effect of platelet-rich plasma improves bladder overactivity in the pathogenesis of ketamine-induced ulcerative cystitis in a rat model. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Xia C., Zhao T., Wang H., Tian H., Xu O., Zhu X., Zhang J., Chen P. Platelet-derived extracellular vesicles ameliorate intervertebral disc degeneration by alleviating mitochondrial dysfunction. Mater. Today Bio. 2023;18 doi: 10.1016/j.mtbio.2022.100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia S.S., Nagwa N.H., Enas S.Z. Evaluating the efficacy of intra-articular injections of platelet rich plasma (PRP) in rheumatoid arthritis patients and its impact on inflammatory cytokines, disease activity and quality of life. Curr. Rheumatol. Rev. 2021;17:232–241. doi: 10.2174/1573397116666201113090629. [DOI] [PubMed] [Google Scholar]
- Dejnek M., Moreira H., Płaczkowska S., Barg E., Reichert P., Królikowska A. Leukocyte-rich platelet-rich plasma as an effective source of molecules that modulate local immune and inflammatory cell responses. Oxidative Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/8059622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong J.M., Russell R.P., Mazzocca A.D. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- Desouza C., Dubey R., Shetty V. Platelet-rich plasma in chronic Achilles tendinopathy. Eur. J. Orthop. Surg. Traumatol. 2023;33:3255–3265. doi: 10.1007/s00590-023-03570-6. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Di Matteo B., Papio T., Tentoni F., Selleri F., Cenacchi A., Kon E., Filardo G. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am. J. Sports Med. 2019;47:347–354. doi: 10.1177/0363546518814532. [DOI] [PubMed] [Google Scholar]
- Dohan Ehrenfest D.M., Rasmusson L., Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Dohan Ehrenfest D.M., Andia I., Zumstein M.A., Zhang C.Q., Pinto N.R., Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4:3–9. [PMC free article] [PubMed] [Google Scholar]
- Dong B., Liu X., Li J., Wang B., Yin J., Zhang H., Liu W. Berberine encapsulated in exosomes derived from platelet-rich plasma promotes chondrogenic differentiation of the bone marrow mesenchymal stem cells via the Wnt/β-catenin pathway. Biol. Pharm. Bull. 2022;45:1444–1451. doi: 10.1248/bpb.b22-00206. [DOI] [PubMed] [Google Scholar]
- Dou H., Wang S., Hu J., Song J., Zhang C., Wang J., Xiao L. Osteoarthritis models: from animals to tissue engineering. J. Tissue Eng. 2023;14 doi: 10.1177/20417314231172584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kadiry A.E.-H., Lumbao C., Salame N., Rafei M., Shammaa R. Bone marrow aspirate concentrate versus platelet-rich plasma for treating knee osteoarthritis: a one-year non-randomized retrospective comparative study. BMC Musculoskelet. Disord. 2022;23:23. doi: 10.1186/s12891-021-04910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes B.T., Enomoto M., Moutos F.T., Carson M.A., Toth J.M., Eggert P., Stallrich J., Willard V.P., Veis D.J., Little D., Guilak F., Lascelles B.D.X. Biological resurfacing in a canine model of hip osteoarthritis. Sci. Adv. 2021;7:eabi5918. doi: 10.1126/sciadv.abi5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts P., Onishi K., Jayaram P., Lana J.F., Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int. J. Mol. Sci. 2020;21:7794. doi: 10.3390/ijms21207794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., Wang X., Jiang W., Zhu Y., Hu Y., Zhao Y., Song X., Zhao J., Zhang W., Peng J., Wang Y. Platelet-rich plasma therapy in the treatment of diseases associated with orthopedic injuries. Tissue Eng. Part B Rev. 2020;26:571–585. doi: 10.1089/ten.teb.2019.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Wang C., Yuan X., Liu Z., Dziak R., Yang S. Combination of controlled release platelet-rich plasma alginate beads and bone morphogenetic protein-2 genetically modified mesenchymal stem cells for bone regeneration. J. Periodontol. 2016;87:470–480. doi: 10.1902/jop.2016.150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca M., Jarak I., Victor F., Domingues C., Veiga F., Figueiras A. Polymersomes as the next attractive generation of drug delivery systems: definition, synthesis and applications. Materials. 2024;17 doi: 10.3390/ma17020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier L.A., Chapman H.S., Pownder S.L., Roller B.L., Cross J.A., Cook J.L., Cole B.J. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am. J. Sports Med. 2016;44:2366–2374. doi: 10.1177/0363546516648644. [DOI] [PubMed] [Google Scholar]
- Freedman B.R., Kuttler A., Beckmann N., Nam S., Kent D., Schuleit M., Ramazani F., Accart N., Rock A., Li J., Kurz M., Fisch A., Ullrich T., Hast M.W., Tinguely Y., Weber E., Mooney D.J. Enhanced tendon healing by a tough hydrogel with an adhesive side and high drug-loading capacity. Nat. Biomed. Eng. 2022;6:1167–1179. doi: 10.1038/s41551-021-00810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuggle N.R., Cooper C., Oreffo R.O.C., Price A.J., Kaux J.F., Maheu E., Cutolo M., Honvo G., Conaghan P.G., Berenbaum F., Branco J., Brandi M.L., Cortet B., Veronese N., Kurth A.A., Matijevic R., Roth R., Pelletier J.P., Martel-Pelletier J., Vlaskovska M., Thomas T., Lems W.F., Al-Daghri N., Bruyère O., Rizzoli R., Kanis J.A., Reginster J.Y. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin. Exp. Res. 2020;32:547–560. doi: 10.1007/s40520-020-01515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Gao L., Groth T., Liu T., He D., Wang M., Gong F., Chu J., Zhao M. Fabrication and properties of an injectable sodium alginate/PRP composite hydrogel as a potential cell carrier for cartilage repair. J. Biomed. Mater. Res. A. 2019;107:2076–2087. doi: 10.1002/jbm.a.36720. [DOI] [PubMed] [Google Scholar]
- Garrasi A., Dodge George R., Kelly John D. Arthroscopic excision of pigmented villonodular synovitis of the trochanteric bursa. Orthopedics. 2023;46:e381–e383. doi: 10.3928/01477447-20230426-02. [DOI] [PubMed] [Google Scholar]
- Geurts J., Jurić D., Müller M., Schären S., Netzer C. Novel ex vivo human osteochondral explant model of knee and spine osteoarthritis enables assessment of inflammatory and drug treatment responses. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19051314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giatromanolaki A., Sivridis E., Athanassou N., Zois E., Thorpe P.E., Brekken R.A., Gatter K.C., Harris A.L., Koukourakis I.M., Koukourakis M.I. The angiogenic pathway “vascular endothelial growth factor/flk-1(KDR)-receptor” in rheumatoid arthritis and osteoarthritis. J. Pathol. 2001;194:101–108. doi: 10.1002/path.842. [DOI] [PubMed] [Google Scholar]
- Gibble J.W., Ness P.M. Fibrin glue: the perfect operative sealant? Transfusion. 1990;30:741–747. doi: 10.1046/j.1537-2995.1990.30891020337.x. [DOI] [PubMed] [Google Scholar]
- Gilbertie J.M., Schaer T.P., Engiles J.B., Seiler G.S., Deddens B.L., Schubert A.G., Jacob M.E., Stefanovski D., Ruthel G., Hickok N.J., Stowe D.M., Frink A., Schnabel L.V. A platelet-rich plasma-derived biologic clears staphylococcus aureus biofilms while mitigating cartilage degeneration and joint inflammation in a clinically relevant large animal infectious arthritis model. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.895022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnacińska M., Małgorzewicz S., Stojek M., Łysiak-Szydłowska W., Sworczak K. Role of adipokines in complications related to obesity: a review. Adv. Med. Sci. 2009;54:150–157. doi: 10.2478/v10039-009-0035-2. [DOI] [PubMed] [Google Scholar]
- Gobbi G., Vitale M. Platelet-rich plasma preparations for biological therapy: applications and limits. Oper. Tech. Orthop. 2012;22:10–15. [Google Scholar]
- Griffin X.L., Smith C.M., Costa M.L. The clinical use of platelet-rich plasma in the promotion of bone healing: a systematic review. Injury. 2009;40:158–162. doi: 10.1016/j.injury.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Guelfi M., Pantalone A., Vanni D., Abate M., Guelfi M.G.B., Salini V. Long-term beneficial effects of platelet-rich plasma for non-insertional Achilles tendinopathy. Foot Ankle Surg. 2015;21:178–181. doi: 10.1016/j.fas.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Guo S.C., Tao S.C., Yin W.J., Qi X., Yuan T., Zhang C.Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81–96. doi: 10.7150/thno.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Paliczak A., Delgado D. Evidence-based indications of platelet-rich plasma therapy. Expert Rev. Hematol. 2021;14:97–108. doi: 10.1080/17474086.2021.1860002. [DOI] [PubMed] [Google Scholar]
- Gupta A., Jeyaraman M., Maffulli N. Common medications which should be stopped prior to platelet-rich plasma injection. Biomedicines. 2022;10 doi: 10.3390/biomedicines10092134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Hu N., Wang C., Ye Z., Wang T., Lan F. Platelet-rich plasma-derived exosomes promote rotator cuff tendon-bone healing. Injury. 2024;55 doi: 10.1016/j.injury.2023.111212. [DOI] [PubMed] [Google Scholar]
- Harrison P. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018;16:1895–1900. doi: 10.1111/jth.14223. [DOI] [PubMed] [Google Scholar]
- Harrison S., Vavken P., Kevy S., Jacobson M., Zurakowski D., Murray M. Platelet activation by collagen provides sustained release of anabolic cytokines. Am. J. Sports Med. 2011;39:729–734. doi: 10.1177/0363546511401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanov E., Fard E.V., Puravath A., Johnston J.S., Peerbhai S., Rojas-Hernandez C.M. T-cell large granular lymphocytic leukaemia in the context of rheumatoid arthritis. Lancet. 2018;392:1071. doi: 10.1016/S0140-6736(18)32208-6. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Akeda K., Yamada J., Kawaguchi K., Takegami N., Fujiwara T., Natsume T., Ide K., Matsuyama Y., Sudo A. Regenerative effects of platelet-rich plasma releasate injection in rabbit discs degenerated by intradiscal injection of condoliase. Arthritis Res. Ther. 2023;25:216. doi: 10.1186/s13075-023-03200-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood L., McWilliams D.F., Pearson C.I., Gill S.E., Ganesan A., Wilson D., Walsh D.A. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- He C., Zheng S., Luo Y., Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Liu A., Yu J., Chen X. Role of platelet-rich plasma gel in promoting wound healing based on medical images of wounds. Contrast Media Mol. Imaging. 2022;2022:1543604. doi: 10.1155/2022/1543604. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hofer M., Lutolf M.P. Engineering organoids. Nat. Rev. Mater. 2021;6:402–420. doi: 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger J.O., Onikepe A.O., MacKrell J., Einhorn T., Bradica G., Lynch S., Hart C.E. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-bb and an injectable beta-tricalcium phosphate/collagen matrix. J. Orthop. Res. 2008;26:83–90. doi: 10.1002/jor.20453. [DOI] [PubMed] [Google Scholar]
- Hou J., Zhang Y., Tang C. New polymer nano-biomaterials in rehabilitation nursing of orthopedic surgery injuries. Mater. Express. 2022;12(175):173–177. [Google Scholar]
- Hou Y., Wen X., Zhou L., Fang X. The value of platelet-rich plasma-derived extracellular vesicles in modern medicine. Ann. Med. 2023;55:2287705. doi: 10.1080/07853890.2023.2287705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgens J.L., Sugg K.B., Grekin J.A., Gumucio J.P., Bedi A., Mendias C.L. Platelet-rich plasma activates proinflammatory signaling pathways and induces oxidative stress in tendon fibroblasts. Am. J. Sports Med. 2016;44:1931–1940. doi: 10.1177/0363546516637176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im H.J., Muddasani P., Natarajan V., Schmid T.M., Block J.A., Davis F., van Wijnen A.J., Loeser R.F. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J. Biol. Chem. 2007;282:11110–11121. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Lee K., Ju J.H. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22052619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiranek W.A., Machado M., Jasty M., Jevsevar D., Wolfe H.J., Goldring S.R., Goldberg M.J., Harris W.H. Production of cytokines around loosened cemented acetabular components. Analysis with immunohistochemical techniques and in situ hybridization. J. Bone Joint Surg. Am. 1993;75:863–879. doi: 10.2106/00004623-199306000-00007. [DOI] [PubMed] [Google Scholar]
- Jones I.A., Togashi R.C., Thomas Vangsness C. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr. Rev. Musculoskelet. Med. 2018;11:558–565. doi: 10.1007/s12178-018-9514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun E.A., Lim K.M., Kim K., Bae O.N., Noh J.Y., Chung K.H., Chung J.H. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology. 2011;5:157–167. doi: 10.3109/17435390.2010.506250. [DOI] [PubMed] [Google Scholar]
- Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbe K., Takagishi K., Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis Rheum. 2002;46:130–137. doi: 10.1002/1529-0131(200201)46:1<130::aid-art10020>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Karan S., Debnath S., Kuotsu K., Chatterjee T.K. In-vitro and in-vivo evaluation of polymeric microsphere formulation for colon targeted delivery of 5-fluorouracil using biocompatible natural gum katira. Int. J. Biol. Macromol. 2020;158:922–936. doi: 10.1016/j.ijbiomac.2020.04.129. [DOI] [PubMed] [Google Scholar]
- Kawabata S., Akeda K., Yamada J., Takegami N., Fujiwara T., Fujita N., Sudo A. Advances in platelet-rich plasma treatment for spinal diseases: a systematic review. Int. J. Mol. Sci. 2023;24:7677. doi: 10.3390/ijms24087677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney R.S., Ji C., Warwick J., Parsons N., Brown J., Harrison P., Young J., Costa M.L., Collaborators A.T.M.T Effect of platelet-rich plasma injection vs sham injection on tendon dysfunction in patients with chronic midportion Achilles tendinopathy: a randomized clinical trial. JAMA. 2021;326:137–144. doi: 10.1001/jama.2021.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevy S.V., Jacobson M.S. Comparison of methods for point of care preparation of autologous platelet gel. J Extra Corpor Technol. 2004;36:28–35. [PubMed] [Google Scholar]
- Khandan-Nasab N., Torkamanzadeh B., Abbasi B., Mohajeri T., Oskuee R.K., Sahebkar A. Application of platelet-rich plasma-based scaffolds in soft and hard tissue regeneration. Tissue Eng. Part B Rev. 2025 doi: 10.1089/ten.teb.2024.0285. [DOI] [PubMed] [Google Scholar]
- Kiaie S.H., Mojarad-Jabali S., Khaleseh F., Allahyari S., Taheri E., Zakeri-Milani P., Valizadeh H. Axial pharmaceutical properties of liposome in cancer therapy: recent advances and perspectives. Int. J. Pharm. 2020;581 doi: 10.1016/j.ijpharm.2020.119269. [DOI] [PubMed] [Google Scholar]
- Kim B.Y., Rutka J.T., Chan W.C. Nanomedicine. N. Engl. J. Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- Kim J., Seo B.-B., Hong K.H., Kim S.E., Kim Y.-M., Song S.-C. Long-term anti-inflammatory effects of injectable celecoxib nanoparticle hydrogels for Achilles tendon regeneration. Acta Biomater. 2022;144:183–194. doi: 10.1016/j.actbio.2022.03.033. [DOI] [PubMed] [Google Scholar]
- Komaki H., Tanaka T., Chazono M., Kikuchi T. Repair of segmental bone defects in rabbit tibiae using a complex of β-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials. 2006;27:5118–5126. doi: 10.1016/j.biomaterials.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Kon E., Filardo G., Delcogliano M., Fini M., Salamanna F., Giavaresi G., Martin I., Marcacci M. Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet. Disord. 2010;11:220. doi: 10.1186/1471-2474-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupratis M.E., Gure A.E., Benson J.M., Ortved K.F., Burris D.L., Price C. Comparative tribology II-Measurable biphasic tissue properties have predictable impacts on cartilage rehydration and lubricity. Acta Biomater. 2022;138:375–389. doi: 10.1016/j.actbio.2021.10.049. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski K., Płomiński J. Gonarthrosis–pathomechanism and diagnosis. Pol. Merkur. Lekarski. 2004;17:415–419. [PubMed] [Google Scholar]
- LaBelle M.W., Marcus R.E. CORR synthesis: what is the role of platelet-rich plasma injection in the treatment of tendon disorders? Clin. Orthop. Relat. Res. 2020;478:1817–1824. doi: 10.1097/CORR.0000000000001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.J., Chau Z.L., Chen S.-Y., Hill J.J., Korpany K.V., Liang N.-W., Lin L.-H., Lin Y.-H., Liu J.K., Liu Y.-C., Lunde R., Shen W.-T. Exosome processing and characterization approaches for research and technology development. Adv. Sci. 2022;9:2103222. doi: 10.1002/advs.202103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana J., Purita J., Paulus C., Huber S.C., Rodrigues B.L., Rodrigues A.A., Santana M.H., Madureira J.L., Jr., Malheiros Luzo Â.C., Belangero W.D., Annichino-Bizzacchi J.M. Contributions for classification of platelet rich plasma - proposal of a new classification: MARSPILL. Regen. Med. 2017;12:565–574. doi: 10.2217/rme-2017-0042. [DOI] [PubMed] [Google Scholar]
- Lansdown D.A., Fortier L.A. Platelet-rich plasma: formulations, preparations, constituents, and their effects. Operat. Tech. Sports Med. 2017;25:7–12. [Google Scholar]
- Lee C.H., Shah B., Moioli E.K., Mao J.J. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Invest. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H., Park H.K., Auh Q.S., Nah H., Lee J.S., Moon H.J., Heo D.N., Kim I.S., Kwon I.K. Emerging potential of exosomes in regenerative medicine for temporomandibular joint osteoarthritis. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Guo P., Klett K., Hall M., Sinha K., Ravuri S., Huard J., Murphy W.L. VEGF-attenuated platelet-rich plasma improves therapeutic effect on cartilage repair. Biomater. Sci. 2022;10:2172–2181. doi: 10.1039/d1bm01873f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Piontek K., Ishida M., Fausther M., Dranoff J.A., Fu R., Mezey E., Gould S.J., Fordjour F.K., Meltzer S.J., Sirica A.E., Selaru F.M. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology. 2017;65:501–514. doi: 10.1002/hep.28735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yang Z., Fang L., Ma C., Zhao Y., Liu H., Che S., Zvyagin A.V., Yang B., Lin Q. Hydrogel Composites with Different Dimensional Nanoparticles for Bone Regeneration. Macromol. Rapid Commun. 2021;42:2100362. doi: 10.1002/marc.202100362. [DOI] [PubMed] [Google Scholar]
- Li W., Pan J., Lu Z., Zhu H., Guo J., Xie D. The application of platelet-rich plasma in the treatment of knee osteoarthritis: a literature review. J. Orthop. Sci. 2022;27:420–428. doi: 10.1016/j.jos.2021.01.006. [DOI] [PubMed] [Google Scholar]
- Li C., Wang J., Yang W., Yu K., Hong J., Ji X., Yao M., Li S., Lu J., Chen Y., Yan S., Wu H., Ma C., Yu X., Jiang G., Liu A. 3D-printed hydrogel particles containing PRP laden with TDSCs promote tendon repair in a rat model of tendinopathy. J. Nanobiotechnol. 2023;21 doi: 10.1186/s12951-023-01892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li L., Wang D., Zhang J., Yi K., Su Y., Luo J., Deng X., Deng F. Fabrication of polymeric microspheres for biomedical applications. Mater. Horiz. 2024;11:2820–2855. doi: 10.1039/d3mh01641b. [DOI] [PubMed] [Google Scholar]
- Liang Y., Li J., Wang Y., He J., Chen L., Chu J., Wu H. Platelet rich plasma in the repair of articular cartilage injury: a narrative review. Cartilage. 2022;13 doi: 10.1177/19476035221118419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.J., Anzaghe M., Schülke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020;9 doi: 10.3390/cells9040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Qi J., Sun Y. Platelet-rich plasma as a potential new strategy in the endometrium treatment in assisted reproductive technology. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Li W., Ni X., Sui Y., Li H., Chen X., Lu Y., Jiang M., Wang C. Growth factors in the treatment of Achilles tendon injury. Front. Bioeng. Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1250533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.H., Srioudom J.R., Sun W., Xing M., Yan S., Yu L., Yang J. The use of hydrogel microspheres as cell and drug delivery carriers for bone, cartilage, and soft tissue regeneration. Biomater. Transl. 2024;5:236–256. doi: 10.12336/biomatertransl.2024.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Min D., Bolton T., Nubé V., Twigg S.M., Yue D.K., McLennan S.V. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care. 2009;32:117–119. doi: 10.2337/dc08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yang Y., Niu X., Lin Q., Zhao B., Wang Y., Zhu L. An in situ photocrosslinkable platelet rich plasma – Complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017;62:179–187. doi: 10.1016/j.actbio.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang L., Ma C., Wang G., Zhang Y., Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2019;14:470. doi: 10.1186/s13018-019-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Awoyemi A.A., Fahy K.E., Thapa P., Borchers C., Wu B.Y., McGlone C.L., Schmeusser B., Sattouf Z., Rohan C.A., Williams A.R., Cates E.E., Knisely C., Kelly L.E., Bihl J.C., Cool D.R., Sahu R.P., Wang J., Chen Y., Rapp C.M., Kemp M.G., Johnson R.M., Travers J.B. Keratinocyte-derived microvesicle particles mediate ultraviolet B radiation–induced systemic immunosuppression. J. Clin. Invest. 2021;131 doi: 10.1172/JCI144963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D.L., Ulici V., Chubinskaya S., Loeser R.F. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is increased in osteoarthritis and regulates chondrocyte catabolic and anabolic activities. Osteoarthr. Cartil. 2015;23:1523–1531. doi: 10.1016/j.joca.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo U.G., Ronga M., Maffulli N. Achilles Tendinopathy. Sports Med. Arthrosc. Rev. 2018;26:16–30. doi: 10.1097/JSA.0000000000000185. [DOI] [PubMed] [Google Scholar]
- López S., Vilar J.M., Sopena J.J., Damià E., Chicharro D., Carrillo J.M., Cuervo B., Rubio M. Assessment of the efficacy of platelet-rich plasma in the treatment of traumatic canine fractures. Int. J. Mol. Sci. 2019;20:1075. doi: 10.3390/ijms20051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo P., Tiziana S., Alberto M., Giovanni Antonio C., Francesco F., Bruno F., Florenzo I., Emilio R. Non-surgical treatment of knee osteoarthritis: multidisciplinary Italian consensus on best practice. Ther. Clin. Risk Manag. 2021;17:507–530. doi: 10.2147/TCRM.S288196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louer C.R., Furman B.D., Huebner J.L., Kraus V.B., Olson S.A., Guilak F. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum. 2012;64:3220–3230. doi: 10.1002/art.34533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovati A.B., Lopa S., Bottagisio M., Talò G., Canciani E., Dellavia C., Alessandrino A., Biagiotti M., Freddi G., Segatti F., Moretti M. Peptide-enriched silk fibroin sponge and trabecular titanium composites to enhance bone ingrowth of prosthetic implants in an ovine model of bone gaps. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.563203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovisolo F., Carton F., Gino S., Migliario M., Renò F. Platelet rich plasma-derived microvesicles increased in vitro wound healing. Eur. Rev. Med. Pharmacol. Sci. 2020;24:9658–9664. doi: 10.26355/eurrev_202009_23055. [DOI] [PubMed] [Google Scholar]
- Lozada J.L., Caplanis N., Proussaefs P., Willardsen J., Kammeyer G. Platelet-rich plasma application in sinus graft surgery: Part I–background and processing techniques. J. Oral Implantol. 2001;27:38–42. doi: 10.1563/1548-1336(2001)027<0038:PPAISG>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Lu A., Miao M., Schoeb T.R., Agarwal A., Murphy-Ullrich J.E. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am. J. Pathol. 2011;178:2573–2586. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., He Z., Shi J., Wang Z., Wu W., Liu J., Kang H., Li F., Liang S. AMD3100 attenuates post-traumatic osteoarthritis by maintaining transforming growth factor-β1-induced expression of tissue inhibitor of metalloproteinase-3 via the phosphatidylinositol 3-kinase/Akt pathway. Front. Pharmacol. 2019;10:1554. doi: 10.3389/fphar.2019.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabey T., Honsawek S., Saetan N., Poovorawan Y., Tanavalee A., Yuktanandana P. Angiogenic cytokine expression profiles in plasma and synovial fluid of primary knee osteoarthritis. Int. Orthop. 2014;38:1885–1892. doi: 10.1007/s00264-014-2406-y. [DOI] [PubMed] [Google Scholar]
- Magalon J., Chateau A., Bertrand B., Louis M., Silvestre A., Giraudo L., Veran J., Sabatier F. DEPA classification: a proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc. Med. 2016;2 doi: 10.1136/bmjsem-2015-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarczyk M.J., Gao Q., He Y., Li Z., Gold M.S., Hochberg M.C., Bunnell B.A., Tuan R.S., Goodman S.B., Lin H. Current models for development of disease-modifying osteoarthritis drugs. Tissue Eng. Part C Methods. 2021;27:124–138. doi: 10.1089/ten.tec.2020.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manole C.G., Soare C., Ceafalan L.C., Voiculescu V.M. Platelet-rich plasma in dermatology: new insights on the cellular mechanism of skin repair and regeneration. Life. 2024;14(40) doi: 10.3390/life14010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markhardt B.K., Huang B.K., Spiker A.M., Chang E.Y. Interpretation of cartilage damage at routine clinical MRI: how to match arthroscopic findings. RadioGraphics. 2022;42:1457–1473. doi: 10.1148/rg.220051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C.E., Smith P.C., Palma Alvarado V.A. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front. Physiol. 2015;6 doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M., Salvador Clavell R., Ródenas Rochina J., Sancho Tello M., Gallego Ferrer G., Gómez Ribelles J.L. Mesenchymal stem cells cultured in a 3D microgel environment containing platelet-rich plasma significantly modify their chondrogenesis-related miRNA expression. Int. J. Mol. Sci. 2024;25:937. doi: 10.3390/ijms25020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mautner K., Malanga G.A., Smith J., Shiple B., Ibrahim V., Sampson S., Bowen J.E. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7:S53–S59. doi: 10.1016/j.pmrj.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Meeson R.L., Todhunter R.J., Blunn G., Nuki G., Pitsillides A.A. Spontaneous dog osteoarthritis - a one medicine vision. Nat. Rev. Rheumatol. 2019;15:273–287. doi: 10.1038/s41584-019-0202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel K.D., Erdmann J.M., McHugh K.P., Abu-Amer Y., Ross F.P., Teitelbaum S.L. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am. J. Pathol. 1999;154:203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton K.K., Barro V., Muller B., Terada S., Fu F.H. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop. J. 2012;32:150–163. [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Harmon K., Woodall J., Vieira A. Sports medicine applications of platelet rich plasma. Curr. Pharm. Biotechnol. 2012;13:1185–1195. doi: 10.2174/138920112800624283. [DOI] [PubMed] [Google Scholar]
- Molinet M., Alves N., Vasconcelos A., Deana N.F. Comparative study of osteoarthritis induced by monoiodoacetate and papain in rabbit temporomandibular joints: macroscopic and microscopic analysis. Folia Morphol. (Warsz) 2020;79:516–527. doi: 10.5603/FM.a2019.0104. [DOI] [PubMed] [Google Scholar]
- Monsel A., Zhu Y.G., Gennai S., Hao Q., Hu S., Rouby J.J., Rosenzwajg M., Matthay M.A., Lee J.W. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto R.R. Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int. 2012;33:379–385. doi: 10.3113/FAI.2012.0379. [DOI] [PubMed] [Google Scholar]
- Mouanness M., Ali-Bynom S., Jackman J., Seckin S., Merhi Z. Use of Intra-uterine Injection of Platelet-rich Plasma (PRP) for endometrial receptivity and thickness: a literature review of the mechanisms of action. Reprod. Sci. 2021;28:1659–1670. doi: 10.1007/s43032-021-00579-2. [DOI] [PubMed] [Google Scholar]
- Mumford A., Frelinger A., Gachet C., Gresele P., Noris P., Harrison P., Mezzano D. A review of platelet secretion assays for the diagnosis of inherited platelet secretion disorders. Thromb. Haemost. 2015;114:14–25. doi: 10.1160/TH14-11-0999. [DOI] [PubMed] [Google Scholar]
- Murakami S., Lefebvre V., de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J. Biol. Chem. 2000;275:3687–3692. doi: 10.1074/jbc.275.5.3687. [DOI] [PubMed] [Google Scholar]
- Murakami M., Nguyen L.T., Zhang Z.W., Moodie K.L., Carmeliet P., Stan R.V., Simons M. The FGF system has a key role in regulating vascular integrity. J. Clin. Invest. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussano F., Genova T., Munaron L., Petrillo S., Erovigni F., Carossa S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets. 2016;27:467–471. doi: 10.3109/09537104.2016.1143922. [DOI] [PubMed] [Google Scholar]
- Myasoedova E. New era for outcomes and management of rheumatoid arthritis: Facing the individualized treatment challenge. Joint Bone Spine. 2021;88 doi: 10.1016/j.jbspin.2020.08.001. [DOI] [PubMed] [Google Scholar]
- Nagae M., Ikeda T., Mikami Y., Hase H., Ozawa H., Matsuda K., Sakamoto H., Tabata Y., Kawata M., Kubo T. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007;13:147–158. doi: 10.1089/ten.2006.0042. [DOI] [PubMed] [Google Scholar]
- Nagashima M., Hasegawa J., Kato K., Yamazaki J., Nishigai K., Ishiwata T., Asano G., Yoshino S. Hepatocyte growth factor (HGF), HGF activator, and c-Met in synovial tissues in rheumatoid arthritis and osteoarthritis. J. Rheumatol. 2001;28:1772–1778. [PubMed] [Google Scholar]
- Namazi H., Mehbudi A. Investigating the effect of intra-articular PRP injection on pain and function improvement in patients with distal radius fracture. Orthop. Traumatol. Surg. Res. 2016;102:47–52. doi: 10.1016/j.otsr.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Nedorubova I.A., Bukharova T.B., Mokrousova V.O., Khvorostina M.A., Vasilyev A.V., Nedorubov A.A., Grigoriev T.E., Zagoskin Y.D., Chvalun S.N., Kutsev S.I., Goldshtein D.V. Comparative efficiency of gene-activated matrices based on chitosan hydrogel and PRP impregnated with BMP2 polyplexes for bone regeneration. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232314720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.H., Kim W., Park K.U., Roh Y.H. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am. J. Sports Med. 2015;43:3062–3070. doi: 10.1177/0363546515608481. [DOI] [PubMed] [Google Scholar]
- Oh J.Y., Kim H.S., Palanikumar L., Go E.M., Jana B., Park S.A., Kim H.Y., Kim K., Seo J.K., Kwak S.K., Kim C., Kang S., Ryu J.H. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018;9:4548. doi: 10.1038/s41467-018-06979-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto S., Nishida K., Yamaai Y., Shibahara M., Nishida T., Doi T., Asahara H., Nakanishi T., Inoue H., Takigawa M. Expression and localization of connective tissue growth factor (CTGF/Hcs24/CCN2) in osteoarthritic cartilage. Osteoarthr. Cartil. 2004;12:771–778. doi: 10.1016/j.joca.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Ong S.Y., Zhang C., Dong X., Yao S.Q. Recent advances in polymeric nanoparticles for enhanced fluorescence and photoacoustic imaging. Angew. Chem. Int. Ed. 2021;60:17797–17809. doi: 10.1002/anie.202101964. [DOI] [PubMed] [Google Scholar]
- Otahal A., Kramer K., Kuten-Pella O., Moser L.B., Neubauer M., Lacza Z., Nehrer S., De Luna A. Effects of extracellular vesicles from blood-derived products on osteoarthritic chondrocytes within an inflammation model. Int. J. Mol. Sci. 2021;22:7224. doi: 10.3390/ijms22137224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- Pan W., Dai C., Li Y., Yin Y., Gong L., Machuki J.O.a., Yang Y., Qiu S., Guo K., Gao F. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials. 2020;239 doi: 10.1016/j.biomaterials.2020.119851. [DOI] [PubMed] [Google Scholar]
- Park H.S., Park J.Y., Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res. Clin. Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Park S.H., Kim D.Y., Lee W.J., Jang M., Jeong S.M., Ku S.K., Kwon Y.S., Yun S. Effect of platelet-rich plasma in Achilles tendon allograft in rabbits. J. Vet. Sci. 2024;25 doi: 10.4142/jvs.23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T., Zhou J., Piepmeier J.M., Saltzman W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 2012;64:701–705. doi: 10.1016/j.addr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y.A., Chen S., Pei M. The essential anti-angiogenic strategies in cartilage engineering and osteoarthritic cartilage repair. Cell. Mol. Life Sci. 2022;79:71. doi: 10.1007/s00018-021-04105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B.Y., Singh A.K., Tsai C.Y., Chan C.H., Deng Y.H., Wu C.M., Chou Y.R., Tsao W., Wu C.Y., Deng W.P. Platelet-derived biomaterial with hyaluronic acid alleviates temporal-mandibular joint osteoarthritis: clinical trial from dish to human. J. Biomed. Sci. 2023;30:77. doi: 10.1186/s12929-023-00962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera J., Delrosso C.A., Nerviani A., Pitzalis C. Clinical phenotypes, serological biomarkers, and synovial features defining seropositive and seronegative rheumatoid arthritis: a literature review. Cells. 2024;13 doi: 10.3390/cells13090743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pest M.A., Russell B.A., Zhang Y.W., Jeong J.W., Beier F. Disturbed cartilage and joint homeostasis resulting from a loss of mitogen-inducible gene 6 in a mouse model of joint dysfunction. Arthritis Rheum. 2014;66:2816–2827. doi: 10.1002/art.38758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp N., Martin H., Juozas B., László T., Rimtautas G., Fiodorovas M., Algimantas Č., Pastucha M., Hoza P., Magos K., Kaywan I., Paša L., Gábor V., Krisztián S., Mohyla M., Farkas C., Oliver K., Kybal S., Robert C.S., Köhler A., Alexandra K., Siegfried T., Christoph G. Treatment of large cartilage defects in the knee by hydrogel-based autologous chondrocyte implantation: two-year results of a prospective, multicenter, single-arm phase III trial. Cartilage. 2022;13 doi: 10.1177/19476035221085146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirri C., Sorbino A., Manocchio N., Pirri N., Devito A., Foti C., Migliore A. Chondrotoxicity of intra-articular injection treatment: a scoping review. Int. J. Mol. Sci. 2024;25:7010. doi: 10.3390/ijms25137010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistone A., Iannazzo D., Espro C., Galvagno S., Tampieri A., Montesi M., Panseri S., Sandri M. Tethering of Gly-Arg-Gly-Asp-Ser-Pro-Lys peptides on Mg-doped hydroxyapatite. Engineering. 2017;3:55–59. [Google Scholar]
- Plachokova A.S., Nikolidakis D., Mulder J., Jansen J.A., Creugers N.H. Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin. Oral Implants Res. 2008;19:539–545. doi: 10.1111/j.1600-0501.2008.01525.x. [DOI] [PubMed] [Google Scholar]
- Pleshko N., Grande D.A., Myers K.R. Nanotechnology in orthopaedics. Am. Acad. Orthop. Surg. 2012;20:60–62. doi: 10.5435/JAAOS-20-01-060. [DOI] [PubMed] [Google Scholar]
- Que W., Liu H., Yang Q. CircPRKCH modulates extracellular matrix formation and metabolism by regulating the miR-145/HGF axis in osteoarthritis. Arthritis Res. Ther. 2022;24:216. doi: 10.1186/s13075-022-02893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni E., Perucca Orfei C., De Luca P., Libonati F., de Girolamo L. Tissue-protective and anti-inflammatory landmark of PRP-treated mesenchymal stromal cells secretome for osteoarthritis. Int. J. Mol. Sci. 2022;23:15908. doi: 10.3390/ijms232415908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi H., Emadi E., Hamidi Alamdari D. Limb saving with a combination of allogenic platelets-rich plasma, fibrin glue, and collagen matrix in an open fracture of the Tibia: a case report. Arch. Bone Jt. Surg. 2023;11:535–538. doi: 10.22038/ABJS.2023.69184.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantapää Dahlqvist S., de Jong B.A.W., Berglin E., Hallmans G., Wadell G., Stenlund H., Sundin U., van Venrooij W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- Rawas-Qalaji M., Cagliani R., Al-Hashimi N., Al-Dabbagh R., Al-Dabbagh A., Hussain Z. Microfluidics in drug delivery: review of methods and applications. Pharm. Dev. Technol. 2023;28:61–77. doi: 10.1080/10837450.2022.2162543. [DOI] [PubMed] [Google Scholar]
- Rayego Mateos S., Rodrigues Diez R., Morgado Pascual J.L., Valentijn F., Valdivielso J.M., Goldschmeding R., Ruiz Ortega M. Role of Epidermal Growth Factor Receptor (EGFR) and its ligands in kidney inflammation and damage. Mediat. Inflamm. 2018;2018:8739473. doi: 10.1155/2018/8739473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboh J.C., Saltzman B.M., Yanke A.B., Fortier L., Cole B.J. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am. J. Sports Med. 2016;44:792–800. doi: 10.1177/0363546515580787. [DOI] [PubMed] [Google Scholar]
- Rodrigues B.L., Montalvão S.A.L., Cancela R.B.B., Silva F.A.R., Urban A., Huber S.C., Júnior J.L.R.C., Lana J.F.S.D., Annichinno-Bizzacchi J.M. Treatment of male pattern alopecia with platelet-rich plasma: a double-blind controlled study with analysis of platelet number and growth factor levels. J. Am. Acad. Dermatol. 2019;80:694–700. doi: 10.1016/j.jaad.2018.09.033. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Merchán E.C. Intra-articular platelet-rich plasma injections in knee osteoarthritis: a review of their current molecular mechanisms of action and their degree of efficacy. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23031301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan C.J., Lohade R.P., Brewer C., Travers J.B. Platelet-activating factor and microvesicle particles as potential mediators for the toxicity associated with intoxicated thermal burn injury. BioFactors. 2022;48:1250–1256. doi: 10.1002/biof.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui S., Yuan Y., Du C., Song P., Chen Y., Wang H., Fan Y., Armstrong D.G., Deng W., Li L. Comparison and investigation of exosomes derived from platelet-rich plasma activated by different agonists. Cell Transplant. 2021;2021:1–13. doi: 10.1177/09636897211017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabarish R., Lavu V., Rao S. A comparison of platelet count and enrichment percentages in the Platelet Rich Plasma (PRP) obtained following preparation by three different methods. J. Clin. Diagn. Res. 2015;9:ZC10–12. doi: 10.7860/JCDR/2015/11011.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghinia A., Davaran S., Salehi R., Jamalpoor Z. Nano-hydroxy apatite/chitosan/gelatin scaffolds enriched by a combination of platelet-rich plasma and fibrin glue enhance proliferation and differentiation of seeded human dental pulp stem cells. Biomed. Pharmacother. 2019;109:1924–1931. doi: 10.1016/j.biopha.2018.11.072. [DOI] [PubMed] [Google Scholar]
- Sample S.J., Racette M.A., Hans E.C., Volstad N.J., Schaefer S.L., Bleedorn J.A., Little J.P., Waller K.R., III, Hao Z., Block W.F., Muir P. Use of a platelet-rich plasma-collagen scaffold as a bioenhanced repair treatment for management of partial cruciate ligament rupture in dogs. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samy T.M., Khater A.H., Ahmed A.K.M. Systematic review of literature on the efficiency of using PRP in arthroscopic rotator cuff repair. QJM Int. J. Med. 2020;113 [Google Scholar]
- Saqlain N., Mazher N., Fateen T., Siddique A. Comparison of single and double centrifugation methods for preparation of Platelet-Rich Plasma (PRP) Pakistan J. Med. Sci. 2023;39:634–637. doi: 10.12669/pjms.39.3.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C., Alt C., Pearce D.A., Furia J.P., Maffulli N., Alt E.U. Methodological flaws in meta-analyses of clinical studies on the management of knee osteoarthritis with stem cells: a systematic review. Cells. 2022;11 doi: 10.3390/cells11060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens F., Clevers H. Human organoids: tools for understanding biology and treating diseases. Annu. Rev. Pathol. 2020;15:211–234. doi: 10.1146/annurev-pathmechdis-012419-032611. [DOI] [PubMed] [Google Scholar]
- Seckin S., Ramadan H., Mouanness M., Kohansieh M., Merhi Z. Ovarian response to intraovarian platelet-rich plasma (PRP) administration: hypotheses and potential mechanisms of action. J. Assist. Reprod. Genet. 2022;39:37–61. doi: 10.1007/s10815-021-02385-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghezzi G., Patel S., Ren C.J., Gualandris A., Pintucci G., Robbins E.S., Shapiro R.L., Galloway A.C., Rifkin D.B., Mignatti P. Fibroblast Growth Factor-2 (FGF-2) induces Vascular Endothelial Growth Factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguin C.A., Bernier S.M. TNFalpha suppresses link protein and type II collagen expression in chondrocytes: role of MEK1/2 and NF-kappaB signaling pathways. J. Cell. Physiol. 2003;197:356–369. doi: 10.1002/jcp.10371. [DOI] [PubMed] [Google Scholar]
- Sharun K., Banu S.A., El-Husseiny H.M., Abualigah L., Pawde A.M., Dhama K., Amarpal Exploring the applications of platelet-rich plasma in tissue engineering and regenerative medicine: evidence from goat and sheep experimental research. Connect. Tissue Res. 2024;65:364–382. doi: 10.1080/03008207.2024.2397657. [DOI] [PubMed] [Google Scholar]
- Shepard J.B., Jeong J.W., Maihle N.J., O’Brien S., Dealy C.N. Transient anabolic effects accompany epidermal growth factor receptor signal activation in articular cartilage in vivo. Arthritis Res. Ther. 2013;15:R60. doi: 10.1186/ar4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoaka T., Ogasawara T., Yonamine A., Chikazu D., Kawano H., Nakamura K., Itoh N., Kawaguchi H. Regulation of osteoblast, chondrocyte, and osteoclast functions by Fibroblast Growth Factor (FGF)-18 in comparison with FGF-2 and FGF-10 *. J. Biol. Chem. 2002;277:7493–7500. doi: 10.1074/jbc.M108653200. [DOI] [PubMed] [Google Scholar]
- Shin H., Jeong S., Lee J.H., Sun W., Choi N., Cho I.J. 3D high-density microelectrode array with optical stimulation and drug delivery for investigating neural circuit dynamics. Nat. Commun. 2021;12:492. doi: 10.1038/s41467-020-20763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shome S., Kodieswaran M., Dadheech R., Chevella M., Sensharma S., Awasthi S., Bandyopadhyay A., Mandal B.B. Recent advances in platelet-rich plasma and its derivatives: therapeutic agents for tissue engineering and regenerative medicine. Progr. Biomed. Eng. 2024;6 doi: 10.1088/2516-1091/ad1338. [DOI] [PubMed] [Google Scholar]
- Singh A.B., Harris R.C. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell. Signal. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Smith J., Sellon J.L. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin. J. Sport Med. 2014;24:88–89. doi: 10.1097/JSM.0000000000000063. [DOI] [PubMed] [Google Scholar]
- Smith W.R., Hudson P.W., Ponce B.A., Rajaram Manoharan S.R. Nanotechnology in orthopedics: a clinically oriented review. BMC Musculoskelet. Disord. 2018;19:67. doi: 10.1186/s12891-018-1990-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Sun R., Wang R., Zhou K., Xie R., Lin J., Georgiev D., Paraschiv A.A., Zhao R., Stevens M.M. Puffball-inspired microrobotic systems with robust payload, strong protection, and targeted locomotion for on-demand drug delivery. Adv. Mater. 2022;34 doi: 10.1002/adma.202204791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar V., Podjaski F., Alapan Y., Kröger J., Grunenberg L., Kishore V., Lotsch B.V., Sitti M. Light-driven carbon nitride microswimmers with propulsion in biological and ionic media and responsive on-demand drug delivery. Sci. Robot. 2022;7:eabm1421. doi: 10.1126/scirobotics.abm1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens K.N., Crespo Biel O., van den Bosch E.E., Dias A.A., Knetsch M.L., Aldenhoff Y.B., van der Veen F.H., Maessen J.G., Stobberingh E.E., Koole L.H. The relationship between the antimicrobial effect of catheter coatings containing silver nanoparticles and the coagulation of contacting blood. Biomaterials. 2009;30:3682–3690. doi: 10.1016/j.biomaterials.2009.03.054. [DOI] [PubMed] [Google Scholar]
- Stilhano R.S., Denapoli P.M.A., Gallo C.C., Samoto V.Y., Ingham S.J.M., Abdalla R.J., Koh T.J., Han S.W. Regenerative effect of platelet-rich plasma in the murine ischemic limbs. Life Sci. 2021;284 doi: 10.1016/j.lfs.2021.119934. [DOI] [PubMed] [Google Scholar]
- Street J., Bao M., deGuzman L., Bunting S., Peale F.V., Ferrara N., Steinmetz H., Hoeffel J., Cleland J.L., Daugherty A., van Bruggen N., Redmond H.P., Carano R.A.D., Filvaroff E.H. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swetledge S., Jung J.P., Carter R., Sabliov C. Distribution of polymeric nanoparticles in the eye: implications in ocular disease therapy. J. Nanobiotechnol. 2021;19 doi: 10.1186/s12951-020-00745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwedowski D., Szczepanek J., Paczesny Ł., Zabrzyński J., Gagat M., Mobasheri A., Jeka S. The effect of platelet-rich plasma on the intra-articular microenvironment in knee osteoarthritis. Int. J. Mol. Sci. 2021;22:5492. doi: 10.3390/ijms22115492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T A., H S., M M., Y K., J H., M H., T S. Vascular endothelial growth factor activates MAP kinase and enhances collagen synthesis in human mesangial cells. Kidney Int. 1999;56:2055–2063. doi: 10.1046/j.1523-1755.1999.00796.x. [DOI] [PubMed] [Google Scholar]
- Tao S.C., Yuan T., Rui B.Y., Zhu Z.Z., Guo S.C., Zhang C.Q. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7:733–750. doi: 10.7150/thno.17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J.A., Tablin F. Activation of equine platelet-rich plasma: comparison of methods and characterization of equine autologous thrombin. Vet. Surg. 2012;41:784–794. doi: 10.1111/j.1532-950X.2012.01016.x. [DOI] [PubMed] [Google Scholar]
- Than A., Liu C., Chang H., Duong P.K., Cheung C.M.G., Xu C., Wang X., Chen P. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat. Commun. 2018;9:4433. doi: 10.1038/s41467-018-06981-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu A. The use of platelet-rich plasma in management of musculoskeletal pain: a narrative review. J. Yeungnam Med. Sci. 2022;39:206–215. doi: 10.12701/jyms.2022.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidnezhad M., Bayer A., Rasuo B., Hock J.V.P., Kweider N., Fragoulis A., Sönmez T.T., Jahr H., Pufe T., Lippross S. Platelet-released growth factors modulate the secretion of cytokines in synoviocytes under inflammatory joint disease. Mediat. Inflamm. 2017;2017:1046438. doi: 10.1155/2017/1046438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S., Liu J., Zhang C. Platelet-rich plasma inhibits inflammatory factors and represses rheumatoid fibroblast-like synoviocytes in rheumatoid arthritis. Clin. Exp. Med. 2017;17:441–449. doi: 10.1007/s10238-017-0449-2. [DOI] [PubMed] [Google Scholar]
- Tong S., Zhang C., Liu J. Platelet-rich plasma exhibits beneficial effects for rheumatoid arthritis mice by suppressing inflammatory factors. Mol. Med. Rep. 2017;16:4082–4088. doi: 10.3892/mmr.2017.7091. [DOI] [PubMed] [Google Scholar]
- Tong L., Yu H., Huang X., Shen J., Xiao G., Chen L., Wang H., Xing L., Chen D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022;10:60. doi: 10.1038/s41413-022-00226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramś E., Malesa K., Pomianowski S., Kamiński R. Role of platelets in osteoarthritis-updated systematic review and meta-analysis on the role of platelet-rich plasma in osteoarthritis. Cells. 2022;11 doi: 10.3390/cells11071080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker J.D., Goetz L.L., Duncan M.B., Gilman J.B., Elmore L.W., Sell S.A., McClure M.J., Quagliano P.V., Martin C.C. Randomized, placebo-controlled analysis of the knee synovial environment following platelet-rich plasma treatment for knee osteoarthritis. PM&R. 2021;13:707–719. doi: 10.1002/pmrj.12561. [DOI] [PubMed] [Google Scholar]
- van Delft M.A.M., Huizinga T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020;110 doi: 10.1016/j.jaut.2019.102392. [DOI] [PubMed] [Google Scholar]
- Van Lieshout E.M.M., Den Hartog D. Effect of platelet-rich plasma on fracture healing. Injury. 2021;52:S58–S66. doi: 10.1016/j.injury.2020.12.005. [DOI] [PubMed] [Google Scholar]
- van Loon J., Macri L. Objective assessment of chronic pain in horses using the Horse Chronic Pain Scale (HCPS): a scale-construction study. Animals (Basel) 2021;11 doi: 10.3390/ani11061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Varela Eirín M., Carpintero Fernández P., Guitián Caamaño A., Varela-Vázquez A., García-Yuste A., Sánchez-Temprano A., Bravo-López S.B., Yañez-Cabanas J., Fonseca E., Largo R., Mobasheri A., Caeiro J.R., Mayán M.D. Extracellular vesicles enriched in connexin 43 promote a senescent phenotype in bone and synovial cells contributing to osteoarthritis progression. Cell Death Dis. 2022;13:681. doi: 10.1038/s41419-022-05089-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatte S., Barton A. Genetics of rheumatoid arthritis susceptibility, severity, and treatment response. Semin. Immunopathol. 2017;39:395–408. doi: 10.1007/s00281-017-0630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volodymyr F., Ruslan Z., Stanislav B., Mandus A. Features of radiological and anatomical changes in bone of the hip joint due to injuries. Ortop. Travmatol. Protez. 2015;7:55–60. [Google Scholar]
- Wang T., He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Wang R., Xu B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021;384:113–127. doi: 10.1007/s00441-020-03319-1. [DOI] [PubMed] [Google Scholar]
- Wang Q., Du Y.-M., Fan L.-H. Structures and properties of chitosan-starch-sodium benzoate blend films. J.-Wuhan Univ. Nat. Sci. Ed. 2003;49:725–730. [Google Scholar]
- Wang Y., Jia M., Zheng X., Wang C., Zhou Y., Pan H., Liu Y., Lu J., Mei Z., Li C. Microvesicle-camouflaged biomimetic nanoparticles encapsulating a metal-organic framework for targeted rheumatoid arthritis therapy. J. Nanobiotechnol. 2022;20:253. doi: 10.1186/s12951-022-01447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Y., Li T., Shen K., Wang K.J., Tian C., Hu D. Adipose mesenchymal stem cell derived exosomes promote keratinocytes and fibroblasts embedded in collagen/platelet-rich plasma scaffold and accelerate wound healing. Adv. Mater. 2023;35 doi: 10.1002/adma.202303642. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li H., Rasool A., Wang H., Manzoor R., Zhang G. Polymeric nanoparticles (PNPs) for oral delivery of insulin. J. Nanobiotechnol. 2024;22(1) doi: 10.1186/s12951-023-02253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J.H., Roberts K.C. Database review of possible factors influencing point-of-care platelet gel manufacture. J Extra Corpor Technol. 2004;36:250–254. [PubMed] [Google Scholar]
- Wei Y., Luo L., Gui T., Yu F., Yan L., Yao L., Zhong L., Yu W., Han B., Patel J.M., Liu J.F., Beier F., Levin L.S., Nelson C., Shao Z., Han L., Mauck R.L., Tsourkas A., Ahn J., Cheng Z., Qin L. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abb3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Li H., Dai H., Hua S., Long X., Li H., Ivanovski S., Xu C. Intra-articular nanoparticles based therapies for osteoarthritis and rheumatoid arthritis management. Mater. Today Bio. 2023;19 doi: 10.1016/j.mtbio.2023.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T., Yi L., Huang J., Luo F., Wen X., Du X., Chen Q., Deng C., Chen D., Chen L. Genetic inhibition of fibroblast growth factor receptor 1 in knee cartilage attenuates the degeneration of articular cartilage in adult mice. Arthritis Rheum. 2012;64:3982–3992. doi: 10.1002/art.34645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.J., Wang Q., MacGregor R.R., Siahaan T.J., Stehno-Bittel L., Berkland C. Adhesion of pancreatic beta cells to biopolymer films. Biopolymers. 2009;91:676–685. doi: 10.1002/bip.21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Li L., Yang Y., Hu P., Li Y., Yang S.Y., Wang L.V., Gao W. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 2019;4:eaax0613. doi: 10.1126/scirobotics.aax0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Piao Y., Liu Q., Yang X. Platelet-rich plasma-derived extracellular vesicles: a superior alternative in regenerative medicine? Cell Prolif. 2021;54 doi: 10.1111/cpr.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.B., Sun N.N., Zhang D., Wang Q., Zhou Q. Efficacy analysis of splint combined with platelet-rich plasma in the treatment of temporomandibular joint osteoarthritis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.996668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Chen Q. Adipokines: new therapeutic target for osteoarthritis? Curr. Rheumatol. Rep. 2019;21:71. doi: 10.1007/s11926-019-0868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Su N., Yang J., Tan Q., Huang S., Jin M., Ni Z., Zhang B., Zhang D., Luo F., Chen H., Sun X., Feng J.Q., Qi H., Chen L. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020;5:181. doi: 10.1038/s41392-020-00222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Zinkle A., Chen L., Mohammadi M. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nat. Rev. Rheumatol. 2020;16:547–564. doi: 10.1038/s41584-020-0469-2. [DOI] [PubMed] [Google Scholar]
- Xu R., Greening D.W., Zhu H.-J., Takahashi N., Simpson R.J. Extracellular vesicle isolation and characterization: toward clinical application. J. Clin. Invest. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Al ani M.K., Sun Y., Xu W., Pan L., Song Y., Xu Z., Pan X., Yang L. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J. Tissue Eng. Regen. Med. 2017;11:1173–1184. doi: 10.1002/term.2020. [DOI] [PubMed] [Google Scholar]
- Xu J., Guangying X., Weiliang Y., Wantao W., Zhuan Z., Wang W. Platelet-rich plasma attenuates intervertebral disc degeneration via delivering miR-141-3p-containing exosomes. Cell Cycle. 2021;20:1487–1499. doi: 10.1080/15384101.2021.1949839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Lin Z., He L., Qu Y., Ouyang L., Han Y., Xu C., Duan D. [Retracted] Platelet-rich plasma-derived exosomal USP15 promotes cutaneous wound healing via deubiquitinating EIF4A1. Oxidative Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/9674809. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yan W., Xu X., Xu Q., Sun Z., Jiang Q., Shi D. Platelet-rich plasma combined with injectable hyaluronic acid hydrogel for porcine cartilage regeneration: a 6-month follow-up. Regenerat. Biomater. 2020;7:77–90. doi: 10.1093/rb/rbz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Xiong W.Q., Li C.Z., Wu M.J., Zhang X.Z., Ran C.X., Li Z.H., Cui Y., Liu B.Y., Zhao D.W. Extracellular vesicles derived from mesenchymal stem cells mediate extracellular matrix remodeling in osteoarthritis through the transport of microRNA-29a. World J. Stem Cells. 2024;16:191–206. doi: 10.4252/wjsc.v16.i2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanxia G., Alice M.W., Ursula F., Malcolm D.S., Mihir D.W., Xiaoli Y., Suzanne C., Carl O., Trudy M., Mary C., Kelly S., Tai-An L., Xuejun L., Susanna P., Douglas J.V., Costantino P., Sunil N. CD40L-dependent pathway is active at various stages of rheumatoid arthritis disease progression. J. Immunol. 2017;198:4490–4501. doi: 10.4049/jimmunol.1601988. [DOI] [PubMed] [Google Scholar]
- Yu X., Wang H., Ning X., Sun R., Albadawi H., Salomao M., Silva A.C., Yu Y., Tian L., Koh A., Lee C.M., Chempakasseril A., Tian P., Pharr M., Yuan J., Huang Y., Oklu R., Rogers J.A. Needle-shaped ultrathin piezoelectric microsystem for guided tissue targeting via mechanical sensing. Nat. Biomed. Eng. 2018;2:165–172. doi: 10.1038/s41551-018-0201-6. [DOI] [PubMed] [Google Scholar]
- Yuan G., Yu C., Du X., Li D., Dou H., Lu P., Wu T., Hao C., Wang Y. Injectable GelMA hydrogel microspheres with sustained release of platelet-rich plasma for the treatment of thin endometrium. Small. 2024;20 doi: 10.1002/smll.202403890. [DOI] [PubMed] [Google Scholar]
- Zahn J., Loibl M., Sprecher C., Nerlich M., Alini M., Verrier S., Herrmann M. Platelet-rich plasma as an autologous and proangiogenic cell delivery system. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/1075975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Wu Y.P., Qian S.J., Teng C., Chen S., Li H. Research progress in the mechanism of effect of PRP in bone deficiency healing. ScientificWorldJournal. 2013;2013 doi: 10.1155/2013/134582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wang M., Gong A., Zhang X., Wu X., Zhu Y., Shi H., Wu L., Zhu W., Qian H., Xu W. HucMSC-exosome mediated-wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- Zhang W., Dong X., Wang T., Kong Y. Exosomes derived from platelet-rich plasma mediate hyperglycemia-induced retinal endothelial injury via targeting the TLR4 signaling pathway. Exp. Eye Res. 2019;189 doi: 10.1016/j.exer.2019.107813. [DOI] [PubMed] [Google Scholar]
- Zhang W., Guo Y., Kuss M., Shi W., Aldrich A.L., Untrauer J., Kielian T., Duan B. Platelet-rich plasma for the treatment of tissue infection: preparation and clinical evaluation. Tissue Eng. Part B Rev. 2019;25:225–236. doi: 10.1089/ten.teb.2018.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Jiang H., Kong Y. Exosomes derived from platelet-rich plasma activate YAP and promote the fibrogenic activity of Müller cells via the PI3K/Akt pathway. Exp. Eye Res. 2020;193 doi: 10.1016/j.exer.2020.107973. [DOI] [PubMed] [Google Scholar]
- Zhang W., Mehta A., Tong Z., Esser L., Voelcker N.H. Development of polymeric nanoparticles for blood-brain barrier transfer-strategies and challenges. Adv. Sci. 2021;8 doi: 10.1002/advs.202003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang X., Chen J., Qian D., Gao P., Qin T., Jiang T., Yi J., Xu T., Huang Y., Wang Q., Zhou Z., Bao T., Zhao X., Liu H., Zheng Z., Fan J., Zhao S., Li Q., Yin G. Exosomes derived from platelet-rich plasma administration in site mediate cartilage protection in subtalar osteoarthritis. J. Nanobiotechnol. 2022;20:733–750. doi: 10.1186/s12951-022-01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yin P., Huang J., Yang L., Liu Z., Fu D., Hu Z., Huang W., Miao Y. Scalable and high-throughput production of an injectable platelet-rich plasma (PRP)/cell-laden microcarrier/hydrogel composite system for hair follicle tissue engineering. J. Nanobiotechnol. 2022;20:465. doi: 10.1186/s12951-022-01671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Zhao F.C., Pang Y., Li D.Y., Yao S.C., Sun S.S., Guo K.J. Downregulation of miR-221-3p contributes to IL-1β-induced cartilage degradation by directly targeting the SDF1/CXCR4 signaling pathway. J. Mol. Med. (Berl) 2017;95:615–627. doi: 10.1007/s00109-017-1516-6. [DOI] [PubMed] [Google Scholar]
- Zhou S., Li L., Chen C., Chen Y., Zhou L., Zhou F.H., Dong J., Wang L. Injectable gelatin microspheres loaded with platelet rich plasma improve wound healing by regulating early inflammation. Int. J. Med. Sci. 2021;18:1910–1920. doi: 10.7150/ijms.51060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P., Wang Z., Sun Z., Liao B., Cai Y. Recombinant platelet-derived growth factor-BB alleviates osteoarthritis in a rat model by decreasing chondrocyte apoptosis in vitro and in vivo. J. Cell. Mol. Med. 2021;25:7472–7484. doi: 10.1111/jcmm.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Zhang Y., Zhou Y. Application progress of modified chitosan and its composite biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23126574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska A., Carreiró F., Oliveira A.M., Neves A., Pires B., Venkatesh D.N., Durazzo A., Lucarini M., Eder P., Silva A.M., Santini A., Souto E.B. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25 doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.