Abstract
Purpose
Cyclin-dependent kinase 4/6 inhibitors (CDK4/6is) combined with endocrine therapy is the recommended first-line (1L) treatment for hormone receptor-positive and human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced breast cancer (ABC). Real-world evidence (RWE) describing 1L CDK4/6i regimens and associated clinical outcomes in Europe is limited. The study objective was to describe clinical characteristics, tumor response, and survival outcomes in patients with HR+/HER2− ABC.
Methods
This retrospective, observational cohort study used data from 52 treatment centers in the UK, Spain, and Germany and included patients who initiated 1L palbociclib + aromatase inhibitor (AI) therapy for ABC between 2016 and 2020. Primary endpoints were real-world progression-free survival (rwPFS) and overall survival (OS).
Results
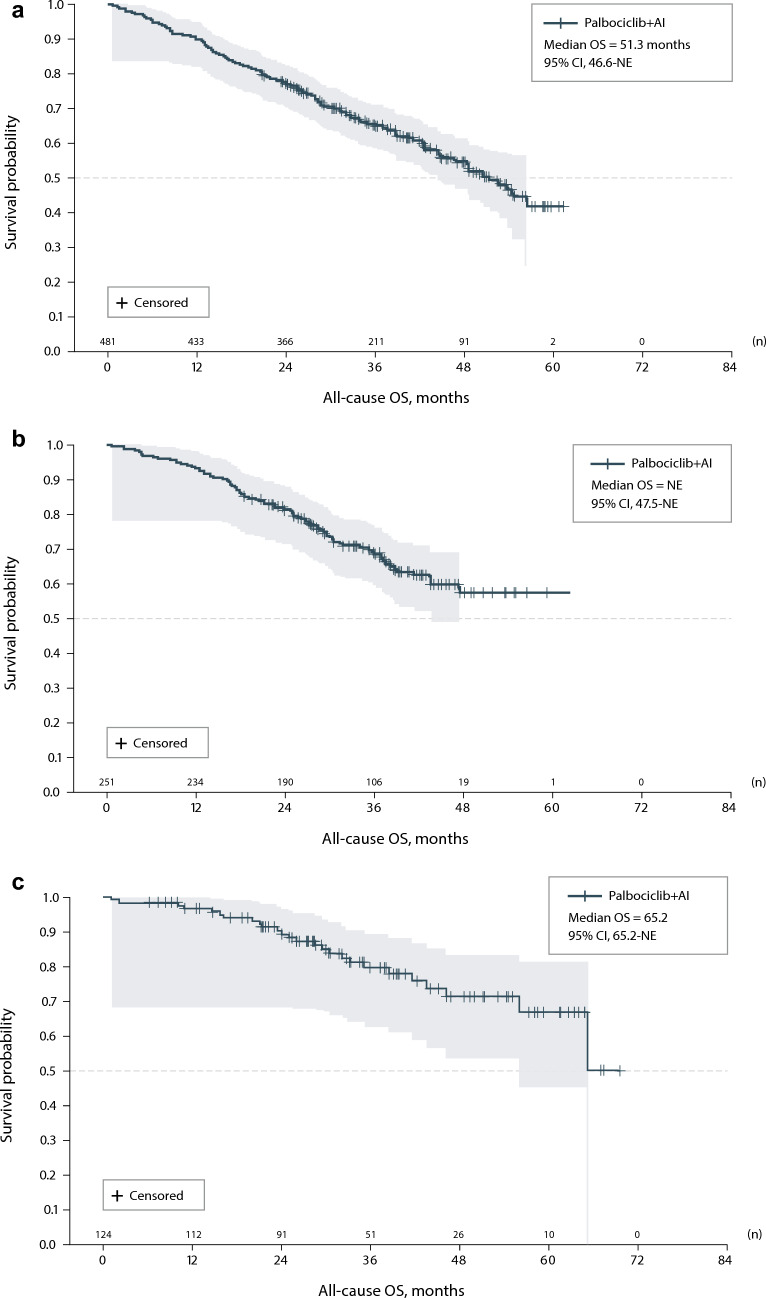
Data were abstracted from 856 patients. During treatment, complete response, partial response, or stable disease was achieved for 86.1% of patients in Spain, 80.7% in the UK, and 79.0% in Germany, while complete or partial response was achieved for 43.8% of patients in Spain, 34.9% in the UK, and 16.9% in Germany. Median rwPFS during treatment was 28.1 months for patients in Spain, 33.9 months in the UK, and 48.1 months in Germany. Median OS was 51.3 months (95% CI 46.6–NE) in the UK, 65.2 months (95% CI 65.2–NE) in Germany, and not reached in Spain.
Conclusion
This RWE supports the clinical effectiveness of 1L palbociclib + AI in routine clinical practice in European countries—consistent with the efficacy observed in clinical trials—and further supports the implementation of palbociclib-based regimens as frontline treatment of HR+/HER2− ABC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-025-07707-5.
Keywords: HR+/HER2− advanced breast cancer, CDK4/6 inhibitor, Palbociclib, Europe, Real-world evidence, Real-world progression-free survival
Introduction
Breast cancer (BC) is the most common cancer diagnosed in women, with over 2.3 million new diagnoses and 665,684 deaths due to BC globally in 2022 [1]. In Europe, BC was both the most frequently diagnosed cancer and most common cause of cancer-related deaths in women, with an estimated 375,000 diagnoses and 96,000 deaths in 2022 [2]. Stage of disease at diagnosis additionally affects prognosis and mortality risk. For example, in the United Kingdom (UK), patients in England diagnosed with later-stage metastatic or advanced BC (ABC) have 5-year survival rates of 26% compared with over 72% for those diagnosed with earlier-stage disease [3]. BC is routinely categorized into subtypes based on expression of hormone receptors (HRs; i.e., estrogen or progesterone receptors) and human epidermal growth factor receptor 2 (HER2), with HR-positive and HER2-negative (HR+/HER2−) tumors representing the most common subtype with a higher relative survival rate than other subtypes [4].
Cyclin-dependent kinase 4/6 inhibitors (CDK4/6is) have revolutionized the treatment landscape for patients with HR+/HER2− ABC since the approval of first-in-class CDK4/6i palbociclib in the United States (US) and Europe [5]. The combination of CDK4/6is like palbociclib with endocrine therapy (ET) such as aromatase inhibitors (AIs) is currently the standard-of-care 1L treatment for patients with HR+/HER2− ABC [6, 7]. Palbociclib is the first-in-class CDK4/6i that, since initial accelerated approval in the United States in 2015 and subsequent approval in Europe in 2016 for use in HR+/HER2− ABC, has been used to treat more than 838,000 patients globally (Data on File Pfizer February 2025 [PP-IBR-GLB-1337]). Randomized clinical trials (RCTs) support the clinical efficacy of adding palbociclib to ET treatment regimens to improve progression-free survival (PFS), first observed in the PALOMA-1 trial, where the addition of palbociclib to letrozole (an AI) for 1L treatment of HR+/HER2− ABC was associated with improved median PFS compared with letrozole alone [8]. Similarly, results of the phase 3 trials PALOMA-2 and PALOMA-3—which assessed palbociclib in combination with letrozole or fulvestrant, respectively—both support improved PFS for combination therapy over ET alone [9, 10]. In both studies, overall survival (OS) was also improved, though not significantly (PALOMA-2, HR = 0.92 [95% CI 0.76–1.12]; PALOMA-3, HR = 0.81 [95% CI 0.64–1.03]) [11, 12].
Real-world evidence (RWE) describing clinical characteristics and outcomes in heterogeneous, real-world populations—including those not included or ineligible for clinical trials—is important to supplement findings from RCTs. Consistent with the PALOMA studies, a retrospective analysis by Rugo et al. [13] of US postmenopausal patients with HR+/HER2− metastatic BC in the Flatiron Health Analytic Database demonstrated an association between palbociclib + AI treatment and improved median real-world PFS (rwPFS) (HR = 0.70 [95% CI 0.62–0.78]) and OS (HR = 0.76 [95% CI 0.65–0.87]) (compared with AI alone). Although 1L CDK4/6i therapy combined with ET is supported by current recommendations in the European Society for Medical Oncology (ESMO) guidelines on treatment for HR+/HER2− ABC, large-scale real-world assessments of characteristics among patients with HR+/HER2− ABC, palbociclib treatment patterns, and associated clinical outcomes in Europe are limited [7, 14, 15]. We present the clinical characteristics and outcomes of patients with HR+/HER2− ABC treated with 1L palbociclib + AI combination therapy in routine clinical practice in the UK, Spain, and Germany.
Materials and methods
Study design
We conducted a multination, retrospective, observational cohort study using data abstracted from medical records. Patients were identified from various treatment centers across the UK, Spain, and Germany. The initial list of treatment centers was selected based on a literature search, previous participation in noninterventional studies, geographic location, and setting type (i.e., cancer center, university/teaching hospital, private hospital/clinic, nonteaching hospital/clinic). Potential sites were administered a systematic feasibility questionnaire, and the final list of participating sites was determined based on the following factors: estimated caseload of eligible patients, treatments typically prescribed for 1L HR+/HER2− ABC, level of access to medical records of deceased and transferred patients, retrospective availability of key data (e.g., vital status, tumor characteristics, treatment details, progression status), electronic data abstraction capability, staff availability, ethics requirements, and contracting timelines [16]. We designed and, where applicable, translated to local languages an electronic case report form (eCRF) for participating treatment center-based research teams to abstract medical record data. Web-based training was provided to the assigned research teams, and questions validating case eligibility were programmed into the eCRF prior to data entry. Ongoing site monitoring was performed to address any site queries.
All eligible patients meeting the criteria and with follow-up data were included. Eligible patients were of female or male sex, aged ≥ 18 years, living or deceased at the time of record abstraction, and diagnosed with advanced (or metastatic) BC that was histologically or cytologically confirmed to be HR+/HER2− subtype. Patients with locally advanced unresectable disease were also eligible. Patients were included if they had a complete medical record covering treatment for ABC that was available for data abstraction and had initiated 1L palbociclib + AI treatment for ABC during the study index period (1 September 2016 to 31 July 2020). Patients were excluded if they had participated in a clinical trial related to treatment of ABC (before or after 1L palbociclib + AI), received prior treatment with any CDK4/6i (i.e., palbociclib, abemaciclib, or ribociclib) in the early-stage BC setting, received retreatment with any CDK4/6i-based regimen following 1L palbociclib + AI (to allow interpretation specifically of 1L palbociclib + AI for HR+/HER2− ABC), or had evidence of active other malignant neoplasms within 3 years prior to ABC diagnosis (with the exception of nonmelanoma skin cancer or carcinoma in situ).
All patients were followed from the study index date (i.e., initiation of 1L palbociclib + AI therapy on or after 1 September 2016) until the earliest of either the last medical record entry or death (Supplemental Fig. S1). To provide a minimum of 12 months of follow-up between treatment initiation and the planned data abstraction date of 31 July 2021 for all patients, study index dates were between 1 September 2016 and 31 July 2020. Patients who died any time after the study index date were included. Data were abstracted from February 2022 through November 2023.
Study measures
We examined demographic and clinical characteristics of patients, including age, sex, race, duration of follow-up, clinical stage at diagnosis, disease-free interval (DFI) between last neoadjuvant or adjuvant treatment and ABC diagnosis, menopausal status for female sex patients, evidence of comorbidities within 12 months of ABC diagnosis, sites of metastases at ABC diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and vital status.
Prior to ABC diagnosis, we assessed treatments (e.g., adjuvant treatment, radiation, surgery). In the ABC setting, we assessed the number of lines of therapy, type of therapy (e.g., chemotherapy, hormonal or targeted therapy), agent composition for each line of therapy up to 3 lines, duration of therapy for each line, palbociclib starting dose and therapy management indicating whether dose was reduced or treatment was interrupted, and reasons for therapy initiation and discontinuation. Clinical outcomes assessed were real-world clinical benefit rate (rwCBR), real-world objective response rate (rwORR), rwPFS, and OS. rwCBR and rwORR were defined as the proportion of patients with documented complete response, partial response, or stable disease (rwCBR), or the proportion with complete or partial response (rwORR) as the best response to treatment, per treatment line. rwPFS was defined per treatment line as time from treatment initiation until earliest of disease progression, death, or date of censoring, where patients without progression events were censored at their last medical record entry date or subsequent line start date; progression was determined by each participating clinician and as documented in patient charts at the time of treatment. OS was defined as time from palbociclib + AI initiation until either all-cause death or date of censoring, where patients alive at data abstraction were censored at last available medical record entry date.
Statistical analysis
Analyses were conducted by individual country. Study measures were summarized descriptively, and the Kaplan–Meier method was used to estimate time-to-event outcomes (i.e., rwPFS and OS) and associated survival rates at selected timepoints (12, 24, 36, and 60 months). Treatment patterns were described for each line of treatment. All analyses were conducted using SAS 9.4 (SAS Studio Version 3.0, SAS Institute).
Supplementary analyses were conducted to evaluate overall survival outcomes using multivariable Cox regression methods, controlling for patient and clinical characteristics: age, ECOG PS, menopausal status, clinical stage at BC diagnosis, visceral versus nonvisceral disease by primary site, bone-only versus other metastatic site(s), DFI, and prior ET. Additional subgroup analyses were conducted by country to evaluate survival outcomes for subgroups by the same covariates.
Results
Study characteristics
Study population
A total of 52 treatment sites were selected, including 18 from the UK, 20 from Spain, and 14 from Germany. Most sites in Spain and about half of the sites in the UK were university or teaching hospitals, while most sites in Germany were private hospitals or clinics. Most sites across countries first utilized a CDK4/6i between 2016 and 2018. From these sites, a total of 856 individuals with HR+/HER2− ABC who had initiated 1L palbociclib + AI treatment met inclusion criteria, including 481 from the UK, 251 from Spain, and 124 from Germany.
Demographic and clinical characteristics
Patient baseline demographic and clinical characteristics are detailed in Tables 1 and 2. Most patients across sites identified as female (98.8–100.0%) and White (75.9–92.8%). Median time to ABC diagnosis from the last treatment received in the neoadjuvant or adjuvant setting ranged from 36.7 to 47.2 months, with a DFI of > 12 months for 52.5–68.8% of patients. At ABC diagnosis, mean (standard deviation [SD]) age ranged from 61.9 (12.8) to 66.6 (12.8) years; notably, 31.8–49.2% of patients were diagnosed with de novo ABC. At 1L treatment initiation, most patients were postmenopausal (UK, 59.5%; Spain, 60.1%; Germany, 78.0%). The ECOG PS at 1L treatment initiation was ≥ 2 for 15.2% of patients in Germany, 16.7% of patients in the UK, and 18.9% of patients in Spain. Mean (SD) duration of follow-up was between 32.2 (12.3) and 34.1 (16.3) months. Most patients (≥ 90%) had at least 1 distant metastatic site at first ABC diagnosis, with frequently observed sites including bone (64.0–72.5%), local lymph nodes (25.8–35.1%), and lung (25.0–30.6%). An average of approximately 2 comorbidities were observed at baseline, most frequently including hypertension (22.9–45.2%), diabetes without end-organ damage (5.6–12.1%), and depression (8.1–11.6%).
Table 1.
Demographic characteristics of patients who received palbociclib + AI as 1L therapy for HR +/HER2− ABC
| UK (n = 481) |
Spain (n = 251) |
Germany (n = 124) |
|
|---|---|---|---|
| Age at ABC diagnosis, mean (SD), years | 63.4 (12.2) | 61.9 (12.8) | 66.6 (12.8) |
| Distribution, n (%) | |||
| < 50 | 67 (13.9) | 50 (19.9) | 14 (11.3) |
| 50 to < 60 | 119 (24.7) | 61 (24.3) | 27 (21.8) |
| 60 to < 70 | 143 (29.7) | 66 (26.3) | 25 (20.2) |
| ≥ 70 | 152 (31.6) | 74 (29.5) | 58 (46.8) |
| Age at initiation of palbociclib + AI, mean (SD) | 63.5 (12.3) | 62.0 (12.8) | 66.7 (12.8) |
| Distribution, n (%) | |||
| < 50 | 66 (13.7) | 50 (19.9) | 14 (11.3) |
| 50 to < 60 | 116 (24.1) | 56 (22.3) | 26 (21.0) |
| 60 to < 70 | 146 (30.4) | 70 (27.9) | 26 (21.0) |
| ≥ 70 | 153 (31.8) | 75 (29.9) | 58 (46.8) |
| Sex, n (%) | |||
| Female | 481 (100.0) | 248 (98.8) | 123 (99.2) |
| Male | 0 (0.0) | 3 (1.2) | 1 (0.8) |
| Race, n (%)a | |||
| Asian | 7 (1.5) | 0 (0.0) | 0 (0.0) |
| Black/African | 7 (1.5) | 0 (0.0) | 0 (0.0) |
| Indian subcontinent | 3 (0.6) | 0 (0.0) | 0 (0.0) |
| Middle Eastern | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White | 365 (75.9) | 233 (92.8) | 108 (87.1) |
| Other: South Europeb | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Don’t know | 101 (21.0) | 18 (7.2) | 16 (12.9) |
| Supplemental private insurance, n (%) | |||
| Yes | 8 (1.7) | 18 (7.2) | 6 (4.8) |
| No | 158 (32.8) | 25 (10.0) | 72 (58.1) |
| Don’t know | 315 (65.5) | 208 (82.9) | 46 (37.1) |
| Vital status at time of medical record abstraction, n (%) | |||
| Alive | 285 (59.3) | 163 (64.9) | 76 (61.3) |
| Deceased | 196 (40.7) | 84 (33.5) | 26 (21.0) |
| Lost to follow-up | 0 (0) | 2 (0.8) | 12 (9.7) |
| Don’t know | 0 (0) | 2 (0.8) | 10 (8.1) |
| Duration of follow-up, monthsc | |||
| N | 481 | 251 | 124 |
| Mean (SD) | 33.2 (14.6) | 32.2 (12.3) | 34.1 (16.3) |
| Median | 33.7 | 32.7 | 32.6 |
| Distribution, n (%) | |||
| 0–6 | 28 (5.8) | 10 (4.0) | 4 (3.2) |
| 7–12 | 26 (5.4) | 9 (3.6) | 10 (8.1) |
| 13–24 | 70 (14.6) | 45 (17.9) | 23 (18.5) |
| 25–36 | 155 (32.2) | 89 (35.5) | 36 (29.0) |
| 37–48 | 126 (26.2) | 81 (32.3) | 26 (21.0) |
| 49–60 | 75 (15.6) | 16 (6.4) | 15 (12.1) |
| ≥ 61 | 1 (0.2) | 1 (0.4) | 10 (8.1) |
1L first line; ABC advanced breast cancer; AI aromatase inhibitor; HER2− human epidermal growth factor receptor 2-negative; HR+ hormone receptor-positive; SD standard deviation; UK United Kingdom
aMultiple responses allowed; thus, rows may add up to greater than 100%
bFor example, Cyprus, Greece, Italy, Spain, Turkey
cLength of follow-up is the duration of time between the study index date and death or end of patient record
Table 2.
Clinical characteristics of patients who received palbociclib + AI as 1L therapy for HR+/HER2− ABC
| UK (n = 481) |
Spain (n = 251) |
Germany (n = 124) |
|
|---|---|---|---|
| Initial BC diagnosis | |||
| Patients with de novo ABC, n (%)a | 153 (31.8) | 103 (41.0) | 61 (49.2) |
| Patients initially diagnosed with stage I, II, IIIA–C, n (%)a | 298 (62.0) | 138 (55.0) | 59 (47.6) |
| Clinical stage at initial BC diagnosis, n (%) | |||
| Stage IA, IB | 56 (11.6) | 36 (14.3) | 13 (10.5) |
| Stage IIA, IIB | 161 (33.5) | 61 (24.3) | 29 (23.4) |
| Stage III: limited regional or locally advanced, resectable with curative intent | 81 (16.8) | 41 (16.3) | 17 (13.7) |
| Stage III: advanced BC not amenable to curative therapy with surgery or radiation | 16 (3.3) | 2 (0.8) | 4 (3.2) |
| Stage IV: metastatic BC | 137 (28.5) | 101 (40.2) | 57 (46.0) |
| Don’t know | 30 (6.2) | 10 (4.0) | 4 (3.2) |
| Time from initial BC diagnosis to ABC diagnosis, na | 298 | 138 | 59 |
| Mean (SD), months | 107.1 (77.8) | 124.0 (85.9) | 114.5 (91.9) |
| DFIb, n (%) | |||
| ≤ 12 months | 115 (38.6) | 41 (29.7) | 28 (47.5) |
| > 12 months | 176 (59.1) | 95 (68.8) | 31 (52.5) |
| Unknown | 7 (2.3) | 2 (1.4) | 0 (0.0) |
| Site(s) of metastases at first diagnosis of ABC, n (%)c,d | |||
| Derived categories of interest | |||
| Bone-only disease | 110 (22.9) | 77 (30.7) | 34 (27.4) |
| Visceral disease | 220 (45.7) | 110 (43.8) | 53 (42.7) |
| Lymph nodes: locale | 169 (35.1) | 81 (32.3) | 32 (25.8) |
| Lymph nodes: distant | 68 (14.1) | 41 (16.3) | 4 (3.2) |
| Bone | 308 (64.0) | 182 (72.5) | 86 (69.4) |
| Liver | 86 (17.9) | 45 (17.9) | 16 (12.9) |
| Lung | 147 (30.6) | 71 (28.3) | 31 (25.0) |
| Pleura | 49 (10.2) | 27 (10.8) | 14 (11.3) |
| Other | 49 (10.2) | 21 (8.4) | 9 (7.3) |
| Total number of distant metastatic sites, n (%) f | |||
| 0 | 43 (9.1) | 4 (1.6) | 8 (6.6) |
| 1 | 205 (43.2) | 128 (52.5) | 64 (52.5) |
| 2 | 129 (27.2) | 64 (26.2) | 39 (32.0) |
| ≥ 3 | 97 (20.5) | 48 (19.7) | 11 (9.0) |
| Time from ABC diagnosis to initiation of 1L treatment, months | |||
| Mean (SD) | 1.6 (1.5) | 1.7 (5.0) | 1.5 (2.1) |
| Performance status at start of 1L ABC treatment, n (%)g | |||
| 0 | 124 (43.1) | 47 (28.7) | 17 (37.0) |
| 1 | 116 (40.3) | 86 (52.4) | 22 (47.8) |
| ≥ 2 e | 48 (16.7) | 31 (18.9) | 7 (15.2) |
| Don’t know | 0 (0) | 0 (0) | 0 (0) |
| Menopausal status at start of 1L ABC treatment (female only), n (%) | |||
| Premenopausal | 133 (27.7) | 85 (34.3) | 11 (8.9) |
| Postmenopausal | 286 (59.5) | 149 (60.1) | 96 (78.0) |
| Don’t know | 62 (12.9) | 14 (5.6) | 16 (13.0) |
| BMI (kg/m2) at ABC diagnosis, n | 322 | 157 | 99 |
| Mean (SD) | 28.4 (6.3) | 27.7 (5.5) | 26.4 (4.8) |
| Total number of comorbidities, mean (SD) | 1.2 (1.3) | 1.4 (1.5) | 1.5 (1.4) |
| NCICI, h mean (SD) | 1.8 (0.8) | 2.0 (0.9) | 2.2 (1.1) |
| Most frequent comorbidities, n (%)d | |||
| Hypertension | 110 (22.9) | 82 (32.7) | 56 (45.2) |
| Diabetes without end-organ damage | 44 (9.1) | 14 (5.6) | 15 (12.1) |
| Depression | 46 (9.6) | 29 (11.6) | 10 (8.1) |
| Chronic pulmonary disease | 24 (5.0) | 1 (0.4) | 8 (6.5) |
| Cerebrovascular disease | 16 (3.3) | 3 (1.2) | 6 (4.8) |
| Other | 171 (35.6) | 111 (44.2) | 42 (33.9) |
| Patient had no past medical history of comorbid conditions | 166 (34.5) | 88 (35.1) | 22 (17.7) |
| Don’t know | 32 (6.7) | 14 (5.6) | 19 (15.3) |
| Family history of BC, n (%) | 89 (18.5) | 69 (27.5) | 16 (12.9) |
1L first line; ABC advanced breast cancer; AI aromatase inhibitor; BC breast cancer; BMI body mass index; DFI disease-free interval; ECOG Eastern Cooperative Oncology Group; HER2− human epidermal growth factor receptor 2-negative; HR+ hormone receptor-positive; NCICI National Cancer Institute’s Combined Index; PS performance status; SD standard deviation; UK United Kingdom
aAmong patients initially diagnosed with early-stage disease
bTime to ABC diagnosis from the last treatment received in the neoadjuvant of adjuvant setting
cCommon responses (> 10% for all countries) included
dMultiple responses allowed; thus, rows may add up to greater than 100%
eAxillary, supraclavicular, and infraclavicular
fExcluding local lymph nodes; among patients whose sites of metastases were known
gAmong patients whose PS was recorded at ABC diagnosis. Assessed via ECOG scale or Karnofsky scores converted to ECOG scale. Score of 2 = ambulatory > 50% of the time and requires occasional assistance, 3 = ambulatory < 50% of the time and requires nursing care, 4 = bedridden, 5 = death
hAmong patients with ≥ 1 NCI comorbidity reported. Calculation does not include cancer as a comorbidity
Treatment patterns
Treatment patterns prior to and following ABC diagnosis are detailed in Table 3. Prior to ABC diagnosis, adjuvant ET was the most frequently observed therapy across countries (74.6–78.3%), and radiation therapy was nearly as common in the UK (72.5%) and Spain (68.8%), but less common in Germany (52.5%). Patients received an average of 2 treatment lines for ABC, including 1L palbociclib + AI. Palbociclib was initiated most commonly at a dose of 125 mg/day (UK, 92.9%; Spain, 95.2%; Germany, 85.5%). Dose was reduced or treatment was temporarily interrupted for 67.8% of patients in the UK, 62.9% of patients in Spain, and 44.4% of patients in Germany. The most frequently observed AI in combination with palbociclib was letrozole, used as the 1L regimen for 83.8% of patients in the UK, 95.2% patients in Spain, and 89.5% of patients in Germany (Supplemental Table S1). At the time of data abstraction, 1L palbociclib + AI treatment was ongoing for 35.8% of patients in the UK, 39.8% of patients in Spain, and 49.2% of patients in Germany. The median (interquartile range) duration of 1L palbociclib + AI therapy among patients who discontinued treatment was 13.8 (6.2–23.8) months in the UK, 15.2 (7.4–24.2) months in Spain, and 16.9 (8.3–25.8) months in Germany, and discontinuations occurred most often due to progressive disease. Between 5.2 and 12.9% of patients did not initiate subsequent treatment, commonly for reasons of frail physical status and death.
Table 3.
Treatment patterns of patients who received palbociclib + AI as 1L therapy for HR+/HER2− ABC
| UK (n = 481) |
Spain (n = 251) |
Germany (n = 124) |
|
|---|---|---|---|
| Duration of 1L treatment for ABCa | |||
| na | 309 | 151 | 63 |
| Median, months | 13.8 | 15.2 | 16.9 |
| Q1, Q3 | 6.2, 23.8 | 7.4, 24.2 | 8.3, 25.8 |
| Palbociclib dosing | |||
| Total daily dose at treatment initiation, n (%) | |||
| 125 mg | 447 (92.9) | 239 (95.2) | 106 (85.5) |
| 100 mg | 23 (4.8) | 8 (3.2) | 16 (12.9) |
| 75 mg | 8 (1.7) | 1 (0.4) | 2 (1.6) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Don’t know | 3 (0.6) | 3 (1.2) | 0 (0.0) |
| Dose reductions or temporary interruptions, n (%) | |||
| Yes | 326 (67.8) | 158 (62.9) | 55 (44.4) |
| No | 126 (26.0) | 89 (35.5) | 51 (41.1) |
| Don’t know | 30 (6.2) | 4 (1.6) | 18 (14.5) |
| Number of patients who discontinued 1L treatment, n (%) | 309 (64.2) | 151 (60.2) | 63 (50.8) |
| Reason(s) for stopping treatment, n (%) b | |||
| Adverse event | 34 (11.0) | 12 (7.9) | 3 (4.8) |
| Patient decision | 23 (7.4) | 5 (3.3) | 6 (9.5) |
| Physician decision | 38 (12.3) | 22 (14.6) | 7 (11.1) |
| Progressive disease | 212 (68.6) | 125 (82.8) | 37 (58.7) |
| No additional clinical benefit expected | 12 (3.9) | 3 (2) | 1 (1.6) |
| Completion of planned course of treatment | 4 (1.3) | 1 (0.7) | 0 (0) |
| Loss to follow-up | 1 (0.3) | 1 (0.7) | 5 (7.9) |
| Death | 27 (8.7) | 8 (5.3) | 4 (6.3) |
| Other | 27 (8.7) | 5 (3.3) | 1 (1.6) |
| Don’t know | 4 (1.3) | 0 (0) | 5 (7.9) |
| Number of patients who did not receive treatment subsequent to 1L, n (%) | 47 (9.8) | 13 (5.2) | 16 (12.9) |
1L first line; ABC advanced breast cancer; AI aromatase inhibitor; CI confidence interval; HER2− human epidermal growth factor receptor 2-negative; HR+ hormone receptor-positive; Q1 first quartile; Q3 third quartile; SD standard deviation; UK United Kingdom
aAmong patients who discontinued 1L treatment
bIn the eCRF, respondents were provided with the options listed as guidance to provide rationale and/or reasoning. Multiple responses were allowed; thus, rows may add up to greater than 100%
Clinical effectiveness and outcomes
Tumor response
Among patients receiving 1L palbociclib + AI, rwCBR was 86.1% (216/251) in Spain, 80.7% (388/481) in the UK, and 79.0% (98/124) in Germany, and rwORR was 43.8% (110/251) in Spain, 34.9% (168/481) in the UK, and 16.9% (21/124) in Germany (Table 4). The best response to treatment across countries was most often a partial response (13.7–35.1%) or stable disease (42.2–62.1%). Notably, patients in Germany were more often observed to have stable disease (62.1%) than partial response (13.7%), while patients in the UK and Spain were more evenly distributed in achieving stable disease (42.2–45.7%) and partial responses (33.5–35.1%).
Table 4.
Tumor response, rwPFS, and OS of patients who received palbociclib + AI as 1L therapy for HR+/HER2− ABC
| UK (n = 481) |
Spain (n = 251) |
Germany (n = 124) |
|
|---|---|---|---|
| Tumor response | |||
| rwCBR,a n (%) | 388 (80.7) | 216 (86.1) | 98 (79.0) |
| rwORR, a n (%) | 168 (34.9) | 110 (43.8) | 21 (16.9) |
| Best response, n (%) | |||
| Complete response | 7 (1.5) | 22 (8.8) | 4 (3.2) |
| Partial response | 161 (33.5) | 88 (35.1) | 17 (13.7) |
| Stable disease | 220 (45.7) | 106 (42.2) | 77 (62.1) |
| Progressive disease | 53 (11.0) | 23 (9.2) | 15 (12.1) |
| Response not assessed | 28 (5.8) | 10 (4.0) | 1 (0.8) |
| Don’t know | 12 (2.5) | 2 (0.8) | 10 (8.1) |
| rwPFSb—all patients | |||
| Event, n (%) | 264 (54.9) | 148 (59.0) | 48 (38.7) |
| Progressed, n (%) | 259 (98.1) | 143 (96.6) | 46 (95.8) |
| Died without progression, n (%) | 5 (1.9) | 5 (3.4) | 2 (4.2) |
| Censored, n (%) | 217 (45.1) | 103 (41.0) | 76 (61.3) |
| Kaplan–Meier estimatesc | |||
| N | 481 | 251 | 124 |
| Median, months | 33.9 | 28.1 | 48.1 |
| 95% CI, months | 28.1–41.1 | 24.6–35.1 | 34.1-NE |
| Progression-free survival rate, % (SE) | |||
| 12 months | 76.6 (2.0) | 75.8 (2.7) | 87.8 (3.0) |
| 24 months | 59.4 (2.3) | 57.0 (3.2) | 75.8 (4.0) |
| 36 months | 48.4 (2.4) | 40.6 (3.4) | 59.0 (5.1) |
| OS—all patients | |||
| Died, n (%) | 196 (40.7) | 84 (33.5) | 26 (21.0) |
| Censored, n (%) | 285 (59.3) | 167 (66.5) | 98 (79.0) |
| Kaplan–Meier estimatesc | |||
| N | 481 | 251 | 124 |
| Median, months | 51.3 | NE | 65.2 |
| 95% CI, months | 46.6-NE | 47.5-NE | 65.2-NE |
| Survival rate, % (SE) | |||
| 12 months | 90.0 (1.4) | 93.2 (1.6) | 96.7 (1.6) |
| 24 months | 77.1 (1.9) | 81.2 (2.5) | 90.3 (2.8) |
| 36 months | 65.4 (2.3) | 68.9 (3.1) | 79.4 (4.2) |
| 60 months | 42.0 (4.6) | 57.2 (4.4) | 66.6 (6.7) |
1L first line; ABC advanced breast cancer; AI aromatase inhibitor; CI confidence interval; DFI disease-free interval; ECOG Eastern Cooperative Oncology Group; ET endocrine therapy; HER2− human epidermal growth factor receptor 2-negative; HR+ hormone receptor-positive; NCICI National Cancer Institute Comorbidity Index; NE not estimable; OS overall survival; PS performance status; rwCBR real-world clinical benefit rate; rwORR real-world overall response rate; rwPFS real-world progression-free survival; SE standard error; UK United Kingdom
aCBR is calculated as the proportion of patients with a documented complete response, partial response, or stable disease; ORR is calculated as the proportion of patients with a documented complete or partial response
bPhysician-assessed disease progression or death were considered “progression events” for progression-free survival calculation. Progression events were documented as the reason for stopping the treatment, best response to treatment, or between the end of treatment and the start of next treatment
cFrom initiation of 1L palbociclib + AI treatment for ABC to progression (rwPFS) or death (OS)
rwPFS
Median rwPFS for patients receiving 1L palbociclib + AI treatment was 48.1 months (95% CI 34.1–not estimable [NE]) for patients in Germany, 33.9 months (95% CI 28.1–41.1) for patients in the UK, and 28.1 months (95% CI 24.6–35.1) for patients in Spain (Table 4, Fig. 1). Across countries, 12-month rwPFS rates were ≥ 75%. rwPFS rates (standard error [SE]) at 36 months were 59.0% (5.1%) in Germany, 48.4% (2.4%) in the UK, and 40.6% (3.4%) in Spain. When adjusting for covariates in the overall population, patients with ECOG PS ≥ 2 or with early-stage disease at initial BC diagnosis (stage I–IIIb) had increased hazard for rwPFS compared with those with PS 0 or 1 or those initially diagnosed with later-stage BC or de novo ABC, respectively; a rwPFS benefit was associated with patients with DFI > 12 months compared with DFI ≤ 12 months (Supplemental Table S2). Similarly, for subgroups by country, patients with ECOG PS ≥ 2, patients with early-stage disease at initial BC diagnosis, and patients in the UK with visceral disease and/or DFI ≤ 12 months tended to have lower rwPFS than respective groups with a PS of 0 or 1, de novo ABC, no visceral disease, or DFI > 12 months (Supplemental Fig. S2). Conversely, patients in the UK and Spain with bone-only disease tended to have better rwPFS than those without.
Fig. 1.
a Kaplan–Meier Analysis of rwPFS—UK. b Kaplan–Meier Analysis of rwPFS—Spain. c Kaplan–Meier Analysis of rwPFS—Germany. AI aromatase inhibitor; rwPFS real-world progression-free survival; UK United Kingdom. Note Shading represents 95% Hall-Wellner Band
OS
Median all-cause OS from initiation of 1L palbociclib + AI was 51.3 months (95% CI 46.6–NE) in the UK, 65.2 months (95% CI 65.2–NE) in Germany, and not reached in Spain (95% CI 47.5–NE) (Table 4, Fig. 2). OS rates (SE) at 36 months were 79.4% (4.2%) in Germany, 68.9% (3.1%) in Spain, and 65.4% (2.3%) in the UK, and 60-month OS rates (SE) were 66.6% (6.7%) in Germany, 57.2% (4.4%) in Spain, and 42.0% (4.6%) in the UK. In line with rwPFS findings, when adjusting for covariates in the overall population, patients with ECOG PS ≥ 2, with visceral disease, or with NCICI score ≥ 3 had increased risk of death compared with respective groups with PS 0 or 1, without visceral disease, or with NCICI score 0 to < 1; an OS benefit was associated with patients with DFI > 12 months compared with DFI ≤ 12 months (Supplemental Table S2). Similarly, for subgroups by country, patients in the UK with an ECOG PS of ≥ 2 or DFI ≤ 12 months tended to have lower OS than comparator groups (Supplemental Fig. S3).
Fig. 2.
a Kaplan–Meier analysis of OS—UK. b Kaplan–Meier Analysis of OS—Spain. c Kaplan–Meier Analysis of OS—Germany. AI aromatase inhibitor; OS overall survival; UK United Kingdom. Note Shading represents 95% Hall–Wellner Band
Discussion
This real-world, population-based study of individuals from the UK, Spain, and Germany with HR+/HER2− ABC receiving 1L palbociclib + AI affirms the clinical effectiveness of palbociclib combination therapy in routine clinical practice and supports the use of palbociclib-based regimens per ESMO guidelines. Median follow-up duration ranged from 32.6 to 33.7 months across countries. Median rwPFS was 48.1 months (95% CI 34.1–NE) for patients in Germany, 33.9 months (95% CI 28.1–41.1) for patients in the UK, and 28.1 months (95% CI 24.6–35.1) for patients in Spain. Median OS was 51.3 months for patients in the UK, 65.2 months for patients in Germany, and not reached for patients in Spain. A complete or partial response was achieved for up to 43.8% of patients receiving 1L palbociclib + AI, and stable disease was observed for approximately 40–60% of patients. In adjusted analyses across countries, worse survival outcomes were associated with ECOG PS ≥ 2 at the start of 1L treatment or DFI ≤ 12 months (rwPFS and OS), as well as with visceral disease or NCICI score ≥ 3 at ABC diagnosis (OS). In subgroup analyses—considering sample sizes were limited—we observed worse survival outcomes for patients with an ECOG PS of ≥ 2 at the start of 1L treatment, as well as patients in the UK with visceral disease at ABC diagnosis and/or DFI ≤ 12 months.
Although comparison across countries was not an objective of this study and differences in characteristics were adjusted for only in supplementary analyses, we observed differences in rwPFS and OS between patients treated with 1L palbociclib + AI in different countries. Median rwPFS was approximately 28 months for patients in Spain and approximately 48 months for patients in Germany, for whom rwORR was lower than that in other countries (16.9% versus 34.9–43.8%). However, over half of patients in Germany achieved stable disease (62.1%), which was not included in the definition of objective response, and rwCBR was similar across countries (79.0–86.1%). Notably, real-world tumor assessments may not be uniform across countries; for instance, progression was assessed for patients in Germany using objective criteria (41.6%) and tumor markers (33.6%) more frequently than in the UK (7.0%; 9.3%), while radiographic evaluations were used in Germany less frequently than in the UK (51.3% versus 95.9%). Moreover, assessment of tumor markers may not be standard practice in certain countries due to accessibility [17]. Though median OS was not reached for the cohort in Spain, 36-month OS rates (SE) were higher for patients in Germany (79.4% [4.2]) than for those in Spain (68.9% [3.1]) and the UK (65.4% [2.3]). Compared with patients in Spain and the UK, greater proportions of patients in Germany were treated in private hospitals/clinics with no hospital beds—an additional healthcare system difference, which may also suggest less severe disease—were postmenopausal, had fewer metastatic sites, and had de novo ABC at baseline, which are characteristics associated with better prognosis [18–22]. The differences we observed in clinical outcomes between countries, within each country, and between clinically relevant covariates and subgroups warrant further investigation.
It is important to supplement findings from RCTs with RWE from heterogenous populations of patients in routine clinical practice who may be ineligible or underrepresented in clinical trials. Progression-free survival improvement upon addition of palbociclib—as well as ribociclib and abemaciclib—to ET is supported by evidence from the respective RCTs assessing each CDK4/6i (PALOMA1 and 2 [8–10], MONALEESA-2 [23], and MONARCH-2 and −3 [24, 25]). This and other real-world studies complement such findings and further support improved PFS: 12-month rwPFS rates observed in this study are similar to 12-month (Spain, 75.8% versus 85.6%; UK, 76.6% versus 85.3%) and 18-month (Germany, 87.8% versus 84.9%) rates from analyses of the European IRIS study [15, 26, 27]. For patients in Spain, the median PFS of 24 months (95% CI 21–27) reported from the PALBOSPAIN study of 762 patients (69.6% received palbociclib + AI) is similar to our observed median PFS of 28.1 months (95% CI 24.6–35.1) [28]. Overall survival results from RCTs assessing 1L CDK4/6i + ET have been mixed; while a significant OS benefit was demonstrated with ribociclib in MONALEESA-2 [23], statistical significance was not reached in respective RCTs assessing palbociclib [11, 12] and abemaciclib [29, 30]. This may be due in part to small sample sizes, differences in trial design, treatment crossover, and missing survival data; furthermore, these findings do not necessarily reflect real-world results for reasons including stringent exclusion and inclusion criteria for patient populations and differences in tumor monitoring and other clinical conditions. This study shows OS at 60 months ranging from 42 to 67%, which supports the clinical effectiveness of palbociclib and is generally in agreement with OS findings from CDK4/6i RCTs (e.g., MONALEESA-2: 60-month OS rate, 52.3% [95% CI 46.5–57.7] [23]) and other real-world studies assessing palbociclib. 24-month OS rates (77.1–90.3%) are similar to those reported for the UK, Spain, and Germany in the European IRIS study (83.2–92.2%) [15]; moreover, the present survival data observed in routine clinical settings in the UK, Germany, and Spain are consistent with findings from the P-REALITY X study in the US (median OS, 49.1 months [95% CI 45.2–57.7]) [13]. Together, this RWE supports real-world effectiveness of palbociclib among a heterogeneous population of patients with HR+/HER2− ABC treated in routine clinical practice.
One key strength of this study is the diversity of the patient population from various treatment sites across 3 European countries. To ensure consistent data collection from treatment sites varying in size, setting, and year of first initial utilization of a CDK4/6i, we conducted a systematic site feasibility assessment, provided training for medical data entry using a standardized eCRF, and conducted periodic site monitoring. The 12-month minimum follow-up opportunity in our study design allowed for estimation of both rwPFS and OS for a broader analysis of palbociclib + AI clinical efficacy. Access to longitudinal data as well as inclusion of patients with complete medical records for treatment of ABC allowed our assessment of treatments used before and after ABC diagnosis up to the 3L of therapy, which provides valuable insights into treatment patterns—including treatment sequencing—for patients with HR+/HER2− ABC in routine clinical practice who initiated treatment with palbociclib + AI.
Nonetheless, we acknowledge our study has limitations, including those inherent to retrospective medical record review studies. Medical records obtained for this study were from sites willing to participate, so findings may not be generalizable to other sites that treat patients with ABC within each country. The number of medical record reviews and data entries that can be feasibly performed by a single participating site may be limited by resource constraints; however, sites were instructed to include all eligible patients. Data captured in our eCRF were limited to information available in the patient’s medical records held by participating sites, and data were entered directly by each site’s research team and therefore may be subject to entry errors and resulting inaccuracies in reporting, although our eCRF includes built-in data and logic checks to improve internal data consistency. Additionally, no comparator arm (i.e., patients receiving AI monotherapy) was included, and sample sizes were limited in each country. Though the primary analysis of rwCBR, rwORR, rwPFS, and OS outcomes was descriptive, clinical practice, study implementation, and site coordinators differences may exist within and between countries. Findings from covariate-adjusted analyses were largely aligned and suggest PS and/or DFI status at the start of 1L treatment and visceral disease status at ABC diagnosis impact survival outcomes. The study population was mainly reported to be White (75.9–92.8%)—with notable proportions of patients (7.2–21.0%) reported to be unknown race—and may therefore not be generally representative of individuals with HR+/HER2− ABC. Moreover, we did not include patients receiving any CDK4/6i in 2L or 3L + therapy; therefore, findings of treatment patterns subsequent to 1L palbociclib + AI are not representative of the whole population of the patients with ABC. However, this permits interpretation of the clinical effectiveness of palbociclib + AI specifically administered as 1L therapy for HR+/HER2− ABC.
Conclusions
This multination, multicenter, retrospective, observational cohort study of individuals from the UK, Spain, and Germany with HR+/HER2− ABC receiving 1L palbociclib + AI in routine clinical practice supports the real-world effectiveness of palbociclib-based regimens per ESMO guidelines. First-line palbociclib + AI therapy was associated with a median PFS range of 28.1–48.1 months and a 60-month OS rate of 42–67% for patients across 3 European countries. This real-world study adds to a growing body of RWE supporting the clinical effectiveness of palbociclib and other CDK4/6i combination therapies for people with HR+/HER− ABC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Gabrielle Dardis, PhD, of RTI Health Solutions for medical writing assistance, and John Forbes of RTI Health Solutions for medical editing assistance. Pfizer, Inc., the sponsor of the study, provided funding for publication support in the form of manuscript writing, styling, and submission.
Author contributions
Conceptualization: RCP, EIB, JD, CC, BL, OO; methodology: RCP, KLD, AH, MIJ, VDA, GF, EIB, JD, CC, BL; formal analysis: RCP, KLD, AH, CC; interpretation: RCP, KLD, AH, VDA, GF, EIB, JD, CC, BL, OO, AW, MJB, EGC; investigation: RCP, KLD, AH, OO, AW, MJB, EGC; supervision: RCP, KLD; project administration: MIJ. All authors reviewed drafts and have read and agreed to the published version of the manuscript.
Funding
This study was sponsored by Pfizer. RCP, MIJ, KLD, AH, VDA, and GF are employees of RTI Health Solutions, an independent nonprofit research organization which was a paid consultant to Pfizer in connection with conduct of the study and development of this manuscript.
Data availability
Data are not publicly available but may be made available on a case-by-case basis, upon request.
Declarations
Competing interests
RCP, MIJ, KLD, AH, VDA, and GF are employees of RTI Health Solutions, an independent nonprofit research organization which was a paid consultant to Pfizer in connection with conduct of the study and development of this manuscript. EIB, JD, CC, and BL are employees and shareholders of Pfizer, Inc., the sponsor of this study. OO is a member of advisory boards for and/or received honoraria from Pfizer, AstraZeneca, Novartis, Exact Sciences, Eisai, Daiichi Sankyo, Roche, Gilead, Eli Lilly, Merck, and Tesaro; received travel grants from Pfizer, AstraZeneca, Novartis, Eisai, Roche, Amgen, Gilead, Eli Lilly, and Menarini; and received research funding from Pfizer, Novartis, Roche, MedDiagnostics, Make2 Count, BCI, and Genomic Health. MJB has no conflicts to report. EG has received consulting fees from Roche, Pfizer, Eli Lilly, Daicchi-Sankyo, Gilead, and AstraZeneca. AW is a member of advisory boards for Amgen, AstraZeneca, Celgene, Eisai, Eli Lilly, Novartis, Pfizer, Roche, Tesaro, Sirtex, MSD, Pierre Fabre, Clovis Oncology, Organon, Seagen, Exact Sciences, Gilead, and Daiichi Sankyo.
Ethics approval and informed consent
The RTI International Institutional Review Board deemed this study exempt from full review. The study was approved by country-specific ethics review boards in Germany, Spain, and the UK. Due to the use of deidentified/pseudonymized data, an informed consent waiver was approved.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74(3):229–263 [DOI] [PubMed] [Google Scholar]
- 2.European Cancer Information System (2022) Breast Cancer in the EU. https://ecis.jrc.ec.europa.eu/pdf/Breast_cancer_2022-Oct_2023.pdf. Accessed 13 Nov 2023
- 3.Office for National Statistics (2019) Cancer survival in England-adults diagnosed. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed. Accessed 13 Nov 2023
- 4.American Cancer Society (2022) Breast cancer facts and figures 2022–2024. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/2022-2024-breast-cancer-fact-figures-acs.pdf. Accessed 13 Nov 2023
- 5.Shah M, Nunes MR, Stearns V (2018) CDK4/6 inhibitors: game changers in the management of hormone receptor-positive advanced breast cancer? Oncology (Williston Park) 32(5):216–222 [PMC free article] [PubMed] [Google Scholar]
- 6.Hui R, de Boer R, Lim E, Yeo B, Lynch J (2021) CDK4/6 inhibitor plus endocrine therapy for hormone receptor-positive, HER2-negative metastatic breast cancer: the new standard of care. Asia Pac J Clin Oncol 17(Suppl 1):3–14 [DOI] [PubMed] [Google Scholar]
- 7.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F et al (2020) 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 31(12):1623–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35 [DOI] [PubMed] [Google Scholar]
- 9.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936 [DOI] [PubMed] [Google Scholar]
- 10.Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H et al (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373(3):209–219 [DOI] [PubMed] [Google Scholar]
- 11.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N et al (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379(20):1926–1936 [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Dieras V, Rugo HS, Harbeck N, Im SA, Gelmon KA et al (2024) Overall survival with palbociclib plus letrozole in advanced breast cancer. J Clin Oncol 42(9):994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugo HS, Brufsky A, Liu X, Li B, McRoy L, Chen C et al (2022) Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2− metastatic breast cancer. NPJ Breast Cancer 8(1):114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis KKS, Last M, Mitra D, Lambert A, Mahtani R (2020) First-line treatment patterns in HR+/HER2− locally advanced or metastatic breast cancer in Europe. Presented at the European Society for Medical Oncology Virtual Congress 2020; 19–21 September 2020. Virtual; 19–21 September 2020. Virtual
- 15.Mycock K, Zhan L, Hart K, Taylor-Stokes G, Milligan G, Atkinson C et al (2022) Real-world treatment of patients with palbociclib for HR+/HER2-advanced/metastatic breast cancer: the Europe IRIS study. Future Oncol 18(3):349–362 [DOI] [PubMed] [Google Scholar]
- 16.Law EH, Galve-Calvo E, Wöckel A, Parikh R, Kurosky SK, Ansquer VD et al (2022) Abstract OT2–19–04: European treatment patterns and outcomes associated with first-line CDK4/6 inhibition and hormonal therapies assessed in a real-world non-interventional study (EUCHARIS). Cancer Res 82(4_Supplement):OT2-19-04-OT2-19–04 [Google Scholar]
- 17.Normanno N, Apostolidis K, Wolf A, Al Dieri R, Deans Z, Fairley J et al (2022) Access and quality of biomarker testing for precision oncology in Europe. Eur J Cancer 176:70–77 [DOI] [PubMed] [Google Scholar]
- 18.Yardley DA, Kaufman PA, Brufsky A, Yood MU, Rugo H, Mayer M et al (2014) Treatment patterns and clinical outcomes for patients with de novo versus recurrent HER2-positive metastatic breast cancer. Breast Cancer Res Treat 145(3):725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH (2010) Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 21(11):2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardia A, Hurvitz S (2018) Targeted therapy for premenopausal women with HR(+), HER2(−) advanced breast cancer: focus on special considerations and latest advances. Clin Cancer Res 24(21):5206–5218 [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Hao Y, Li N, Lin PL, Ohashi E, Koo V et al (2015) Clinical outcomes among HR+/HER2− metastatic breast cancer patients with multiple metastatic sites: a chart review study in the US. Exp Hematol Oncol 4:31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuyun Carter G, Mohanty M, Stenger K, Morato Guimaraes C, Singuru S, Basa P et al (2021) Prognostic factors in hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced breast cancer: a systematic literature review. Cancer Manag Res 13:6537–6566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L et al (2022) Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med 386(10):942–950 [DOI] [PubMed] [Google Scholar]
- 24.Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X et al (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884 [DOI] [PubMed] [Google Scholar]
- 25.Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T et al (2019) MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mycock K, Hanson KA, Taylor-Stokes G, Milligan G, Atkinson C, Mitra D et al (2022) Real-world treatment patterns and clinical outcomes associated with palbociclib combination therapy: a multinational, pooled analysis from the ibrance real world insights study. Clin Ther 44(12):1588–1601 [DOI] [PubMed] [Google Scholar]
- 27.Taylor-Stokes G, Zhan L, Mycock KL, Milligan G, Ghale A, Mitra D (2020) 177P Real world treatment patterns and clinical outcomes associated with palbociclib combination therapy in Germany: Results from the IRIS study. Ann Oncol 31:S79–S80 [Google Scholar]
- 28.Martínez-Jáñez N, Ezquerra MB, Henao F, Manso L, Antón A, Zamora P et al (2023) Abstract P4–01–28: Palbospain: observational analysis of first-line therapy with palbociclib in patients with HR+/HER2− metastatic breast cancer (MBC) In Real-Life Conditions. Cancer Res 83(5):P4-01-28-P4-01–28 [Google Scholar]
- 29.Goetz MP, Toi M, Huober J, Sohn J, Tredan O, Park IH et al (2024) Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2− advanced breast cancer: final overall survival results of MONARCH 3. Ann Oncol 35:1202–1204 [DOI] [PubMed] [Google Scholar]
- 30.Grinshpun A, Tolaney SM, Burstein HJ, Jeselsohn R, Mayer EL (2023) The dilemma of selecting a first line CDK4/6 inhibitor for hormone receptor-positive/HER2-negative metastatic breast cancer. NPJ Breast Cancer 9(1):15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not publicly available but may be made available on a case-by-case basis, upon request.