Fig. 5.
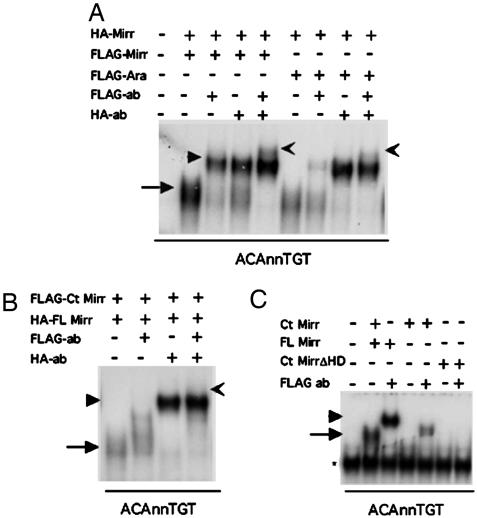
Iro proteins form homodimers and heterodimers in vitro. (A) When FLAG-tagged and HA-tagged Mirr are cotranslated and mixed with labeled ACAnnTGT probe, a DNA-protein shift is formed (arrow). Addition of each of the Abs against the two different tags results in a supershift (arrowheads). Addition of both Abs in the same reaction results in a super-supershift (notched arrow), indicating that the complexes contain both FLAG and the HA-tagged Mirr. The same is shown for HA-Mirr and FLAG Ara indicating that Mirr can form heterodimers with other Iro proteins in vitro. (B) When an N-terminal deletion construct is cotranslated with Mirr and mixed with the ACAnnTGT probe, no super-supershift is detected, suggesting that sequences in the N-terminal region are important for dimerization. (C) The N-terminal deleted Mirr binds DNA weakly, and a complex can only be detected upon addition of the Ab.