Abstract
Background:
In the United States, spillover of West Nile virus (WNV) into wild mammal populations has been reported since the introduction of the virus into the New World in 1999. Eastern gray squirrels (Sciurus carolinensis) exhibit a high seroprevalence for WNV in urban settings where high virus circulation and human spillover have been reported. In Atlanta, Georgia, human cases of WNV are uncommon despite high infection rates in birds and mosquitoes. In this study, we evaluated WNV exposure of eastern gray squirrels in a WNV hot spot in Atlanta.
Materials and Methods:
Gray squirrels were live-trapped in Grant Park, Atlanta, during July–October, 2012, and a census was conducted to estimate squirrel density in the study site. Sera from trapped animals were tested for circulating virus-by-virus isolation in cell culture and for WNV-specific antibodies by enzyme-linked immunosorbent assay and plaque reduction neutralization test. Mosquitoes were collected at the same location and tested for virus isolation.
Results:
Among the 69 collected squirrels, 25 (36.2%) tested positive for WNV antibodies, although none were viremic. Seroprevalence was lower in juveniles (18.8%) than in adults (37.5%), but this difference was not statistically significant. Gender and squirrel density had no effect on seroprevalence. Seasonality of squirrel seroprevalence and of mosquito infection were significantly associated, both peaking in August. No difference in squirrel exposure was detected across the collection sites.
Conclusions:
We report a high degree of WNV exposure in squirrels in Grant Park that was correlated with seasonality of mosquito infection. The detection of antibodies in juveniles suggests that circulation of WNV in the surveyed population is ongoing. Eastern gray squirrels may be suitable indicators for virus amplification and for risk of human spillover on a local scale in urban settings.
Key Words: : West Nile Virus, Gray squirrels, Arbovirus(es), Zoonosis, Culex, Rodents
Introduction
West Nile virus (WNV) was first detected in the United States in late summer of 1999. Over the subsequent decade, the virus has spread rapidly throughout North America and is now considered endemic (Kramer et al. 2008, Mann et al. 2013, Petersen et al. 2013). WNV is maintained in an enzootic cycle between birds and ornithophilic mosquitoes, primarily from the genus Culex (Kramer et al. 2008, Mann et al. 2013, Petersen et al. 2013). In humans, horses, and some bird species (e.g., corvids, blue jays), WNV infection can result in meningitis, encephalitis, permanent neurological impairment, and death (Kramer et al. 2008, Mann et al. 2013, Petersen et al. 2013).
WNV exposure has been detected in many other mammals, such as bats, raccoons, opossum, rabbit, mice, skunks, and tree squirrels (Dietrich et al. 2005, Root et al. 2005, Gómez et al. 2008a, Blitvich et al. 2009). Most mammalian species are considered dead-end hosts because the viremia levels following exposure are not high enough to infect the vector (Bunning et al. 2002, Ratterree et al. 2004, Read et al. 2005). Over the last decade, several studies performed in both laboratory conditions and natural settings re-examined whether specific mammals are noncompetent hosts. Studies of viremia in cottontail rabbits, eastern chipmunks, golden hamsters, and tree squirrels demonstrated that these species presented viremia above the threshold required to infect Culex mosquitoes (Tesh et al. 2005, Tiawsirisup et al. 2005, Root et al. 2006, Platt et al. 2007, Gómez et al. 2008b).
Field studies have documented that tree squirrels and chipmunks are commonly exposed to WNV with mean population seroprevalence comparable to levels reported in bird populations (Dietrich et al. 2005, Root et al. 2005, Gómez et al. 2008a, Blitvich et al. 2009). In squirrels, laboratory experiments detected virus in blood up to 5 days postinoculation, with a viremia peak of 104.98 plaque-forming units (pfu)/mL. WNV has also been isolated from fecal and urine samples obtained from infected squirrels up to 20 days after initial infection (Root et al. 2006, Gómez et al. 2008b).
Urban green spaces are favorable habitats for sciurids (e.g., tree squirrels and chipmunks), which typically form big colonies in these settings, sharing this urban environment with mosquito vectors, birds, and humans. The home range of squirrels is limited compared to that of birds, and, thus, they may indicate the potential for virus spillover to mammals on a local scale (Don 1983, Koprowski 1994, Whitaker and Hamilton 1998). Eastern gray squirrels may be a useful indicator of local virus transmission because they breed twice annually, and seroprevalence in juveniles can be used to detect virus transmission during the WNV transmission season (Whitaker and Hamilton 1998). Furthermore, the levels of viremia and seroprevalence in sciurids suggest that they also may indicate risk of WNV spillover to humans and may even play a role in WNV transmission.
In Georgia, although rates of seroprevalence in bird reservoirs and of mosquito infection are similar to those observed in areas with high levels of human spillover (Gibbs et al. 2006, Vazquez-Prokopec et al. 2010), the number of reported human cases is relatively low (Centers for Disease Control and Prevention 2014). Recently, WNV hot spots have been identified in the Atlanta metropolitan area (Vazquez-Prokopec et al. 2010) in and around wooded areas (the city of Atlanta is known as the “city in the forest” because of its high tree coverage). This type of landscape favors high abundance of tree squirrels (Sciurus carolinensis), which, given their arboreal behavior (squirrels usually build their nests at the fork of tree branches or inside tree holes), share their habitat with birds. There is also a positive correlation between tree coverage and a high abundance of Culex (Cx.) quinquefasciatus in Atlanta (Vazquez-Prokopec et al. 2010), which may increase the amount of contact between mosquitoes and tree squirrels and therefore, the risk of WNV spillover.
Here, we report the results of a study targeting squirrels to help explain the relatively low number of reported human cases in Georgia (Centers for Disease Control and Prevention 2014, Ruiz et al. 2004, 2007). We focused on the possible role of squirrels in WNV transmission in Grant Park, an urban park in Atlanta. In this area, there has been reported evidence of spillover of WNV to humans (Vazquez-Prokopec et al. 2010), and WNV has been detected in birds and mosquitoes in and around Grant Park annually since 2001 (D.G. Mead, personal communication). We also examined whether WNV seroprevalence in tree squirrels could be used as an indicator for local-scale WNV transmission risk to humans in an urban setting.
Materials and Methods
Study area
Between June and October of 2012, we captured squirrels and collected mosquitoes in Grant Park, Atlanta, GA. This park has high tree density and is heavily frequented by humans. The study area has described earlier (Levine et al. 2013), and WNV infection in birds has been documented (Levine et al. 2013).
Squirrel trapping and processing
We collected specimens in seven sites with a mean distance of 150 meters between sites. Trapping was performed biweekly using five Tomahawk traps (48.33×15.23×15.2 cm; Tomahawk Live Trap, Tomahawk, WI) in an area of ∼50 meters2 per site for a total of 280 trap/days. Traps were baited with peanut butter and a mixture of seeds. At each site, the five traps were set approximately 5 meters apart, and opened during mornings (6:00 to 12:00) or afternoons (16:00 to 20:00), periods that correspond to the daily peaks of squirrel activity. Because the park is heavily used, we checked the traps every 45 min to ensure that they were not disturbed.
All captured squirrels were anesthetized according to published guidelines (Parker et al. 2008) by inhalation of isoflurane (Iso Flo1, Abbott Laboratories, North Chicago, IL). We aged each animal as adult or juvenile based on size, fur color, and breeding attributes (Koprowski 1994). Blood samples were collected from the femoral vein, transported on ice to the laboratory, and centrifuged at 10,000 rpm for 30 min. After centrifugation, the serum was removed and stored at −80°C for further processing. All procedures were approved by Emory University IACUC committee (DAR-2001178-051515B, approved 5/15/2012).
Squirrel density estimation method
To evaluate squirrel density, two squirrel censuses were conducted at the beginning (early July, 2012) and the end (late September, 2012) of the trapping season. We applied area counts to estimate squirrel density in our study (Hein 1997, Steele and Koprowski 2003). We divided the study area into 30 equal-sized quadrants of 100 meters2 and established a vantage point within each quadrant, from which each operator started the surveillance facing north and covered the quadrant turning clockwise. Each quadrant was observed for 15 min, and the operator recorded the distance and direction of each sighted squirrel from the vantage point.
The sampling order of each area was predetermined using random number generation (Flyger 1959, Parker and Nilon 2008). Counts were conducted in each area from sunrise to 2 h after sunrise and 2 h prior to sunset until sunset, which corresponds to peak squirrel activity in urban areas (Manski et al. 1981, Gustafson and VanDruff 1990). To reduce the probability of count bias due to disturbance factors, we visited each quadrant both in mornings and evenings on different days. Density was calculated as described by Flyger (1959).
Mosquito collection and processing
Adult mosquitoes were collected using CDC gravid traps (Reiter et al. 1986) baited with a mixture of dog food and hay infusion. One trap per site was set night (from 18:00 to 8:00) before the squirrel-trapping session. Collected female mosquitoes were identified to genus (Slaff and Apperson 1989), sorted into pools of up to 25 (according to species, trap site, and collection date), and stored at −80°C. Mosquito pools were homogenized using a Qiagen Mixer Mill 300 (Valencia, CA) and clarified by centrifugation (10 min at 9000 rpm) for virus isolation performed at the Southeastern Cooperative Wildlife Disease Study at the University of Georgia (Athens, GA).
Enzyme-linked immunosorbent assay
Sera were screened at a dilution of 1:10 for antibodies to flaviviruses by epitope-blocking enzyme-linked immunosorbent assay (ELISA), as described previously (Blitvich et al. 2003, 2009). Briefly, ELISAs were performed using the WNV-specific monoclonal antibody (mAb) 3.1112G (Chemicon International, Temecula, CA) or the flavivirus group-reactive mAb 6B6C-1 (InBios International, Seattle, WA). Antigen was prepared from WNV-infected Aedes albopictus (C6/36) cell cultures. The ability of the test sera to block the binding of the mAbs to WNV antigen was compared with the blocking ability of control serum without antibody to flaviviruses. Data were expressed as relative percentages, and inhibition values ≥30% were considered as indicating the presence of viral antibodies.
Plaque reduction neutralization test
All sera positive for flavivirus antibodies by blocking ELISA were further tested by plaque reduction neutralization test (PRNT) to identify the infecting virus. PRNTs were conducted according to standard methods in the Biosafety Level 3 facilities at Iowa State University (Beaty et al. 1995) using WNV (strain NY99–35261–11) and St. Louis encephalitis virus (SLEV; strain TBH-28). SLEV was included because it has been identified in Georgia and is known to react with antibodies to WNV (Calisher et al. 1989, Gubler et al. 2007). Sera were initially tested at a dilution of 1:10, and those that tested positive were further diluted and tested to determine their end-point titers. Titers were expressed as the reciprocal of highest serum dilutions yielding ≥90% reduction in the number of plaques (PRNT90). For etiologic diagnosis, the PRNT90 antibody titer to the respective virus needed to be at least four-fold greater than that shown by the other tested flavivirus.
Virus isolation in cell culture
All squirrel sera and mosquito homogenates were tested for cytopathic virus by performing virus isolation in Vero Middle America Research Unit (MARU, Vero M) cells. Cell cultures were examined regularly for evidence of cytopathic effect (CPE). CPE-positive cultures were tested for WNV using VecTest® strips (Medical Analysis Systems, Inc., Camarillo, CA). Reverse transcription polymerase chain reaction (RT-PCR) was performed to confirm the presence of WNV RNA in VecTest-positive samples using WNV-specific primers as described by Levine et al. (2013). The minimum infection rate (MIR) was calculated using maximum likelihood (ML) (Bustamante and Lord 2010) and is expressed as the number of positive mosquito pool per 1000 tested mosquitoes.
Statistical analysis
The Fisher least significant difference (LSD) test (Conover 1998) was used to evaluate the differences between the proportion of seropositive squirrels by study site, age group (adult/juvenile), and gender. The Fisher LSD test was also applied to test for significant differences between mosquito MIR by site. Differences in abundance of collected mosquitoes between months were compared using a Mann–Whitney–Wilcoxon test.
The effect of squirrel gender, age, study site, and monthly MIR on animal seropositivity was tested using a logistic regression based on a generalized linear model (GLM). Starting from a full model formula:
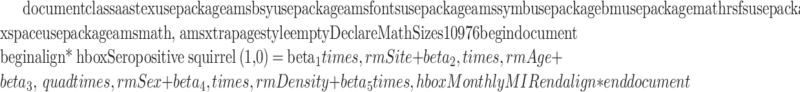 |
We applied a multimodel selection approach to find the best model(s) using Akaike information criteria (AIC) (Burnham and Anderson 2002). We calculated the ΔAIC as the difference between each model and the one with the lowest AIC value. All models with a ΔAIC <2 were included in the set of best models (Burnham and Anderson 2002). A cumulative link mixed models (CLMM) procedure was performed to investigate the relationship of antibody titer (ordered categorical data) in seropositive squirrels with specimen characteristics and capture month. In the CLMM model, sampling sites were set as a random effect to adjust for environmental components and pseudo-replication.
Results
Squirrel census and collection
The density of squirrels for the study area was determined to be 18.9/ha, with a range of 14.8–22/ ha (Table 1). During our study, we collected 69 individuals with three (4.2%) recaptures. All three recaptured squirrels were retrapped at their original trapping site, suggesting a limited movement range. Of the 69 individuals, 44 were males (61.4%) and 16 were juveniles (22.8%). Thirty-one squirrels (44.8%) showed signs of breeding attributes (e.g., testicular dimension, vagina shape, and pregnancy). The lowest number of squirrels was captured in July (Fig. 1), when the collections were affected by consecutive rain events within a short time period (data not shown), and we were not able to set traps until the 18th of the month.
Table 1.
Squirrel and Mosquito Abundance, Seroprevalence, and Minimum Infection Rate
| Site | No. of Culex pools (n mosquitoes) | MIR (n positive pools) (95% CI) | Captured squirrels (density/ha) | Seroprevalemce (n positive) (95% CI) |
|---|---|---|---|---|
| Site A | 17 (102) | 9.8 (1) (0.2; 53.4) |
11 (22.0) | 45.5% (5) (16.8–76.6%) |
| Site_B | 40 (558) | 7.1 (4) (1.9; 18.2) |
10 (16.5) | 30.0% (3) (6.7–65.2%) |
| Site_C | 26 (243) | 4.1 (1) (0.1; 22.7) |
9 (20.6) | 33.3% (3) (7.4–70.1%) |
| Site D | 44 (561) | 8.9 (5) (2.9; 20.6) |
3 (14.8) | 100% (3) (29.2–100%) |
| Site E | 31 (323) | 6.1 (2) (0.7; 22.2) |
3 (21.2) | 33.3% (1) (0.8–90.5%) |
| Site F | 30 (351) | 2.8 (1) (0.1; 15.7) |
18 (20) | 38.9% (7) (17.3–64.3%) |
| Site G | 120 (1850) | 2.7 (5) (0.8; 6.2) |
15 (19.5) | 13.3% (2) (1.6–40.4%) |
| All sites | 308 (3988) | 4.7 (19) (2.8; 7.4) |
69 (18.9) | 34.7% (24) (23.7–47.2%) |
MIR, mnimum infection rate; CI, confidence interval.
FIG. 1.
Collections of female mosquitoes and squirrels, June–October, 2012. (A) Median number and interquartile interval of Culex spp. female mosquitoes collected per gravid trap. (B) Number of adult (Adl) and juvenile (Juv) squirrels captured using live traps.
We collected a total of 72 sera from the 69 squirrels. Twenty-five (36.2%; 95% confidence interval [CI] 24.9–48.6%) sera tested positive for WNV antibodies (Table 1). Among the seropositive individuals, three (12%) were juveniles. Seroprevalence of the adult group (37.5%; 95% CI 24.9–51.5%) was higher than in juveniles (18.8%; 95% CI 4.1–45.6%), but this difference was not significant (odds ratio [OR]=2.7; 95% CI 0.61–15.68; p>0.05). Seroprevalence in males (32.6%; 95% CI 19.1–48.5%) and females (34.5%; 95% CI 17.9–54.3%) was not significantly different (OR=1.09; 95% CI 0.35–3.29, p>0.05). No significant differences (OR=2.33; 95% CI 0.77–7.25; p>0.05) were detected between the seroprevalence of squirrels showing breeding signs (44.8%; 95% CI 26.4–64.3%) and nonbreeding individuals (25.6%; 95% CI 13.5–41.2%). A significant difference (Fisher test, p<0.05) was found between the number of seropositive squirrels in August, when prevalence peaked (70.6%; 95% CI 44–89.6%), and July when none of the individuals captured were seropositive (0%; 95% CI 0–33.6%).
WNV PRNT90 titers of positive squirrels ranged from 40 to 1280 (Fig. 2). Only squirrels collected in the summer had antibody titers higher than 320 (Fig. 2), and the highest value of antibody titer (1280) was recorded in an adult squirrel in June. Juvenile squirrels had antibody titers ranging from 160 to 640, and the highest value for this age group was recorded in August (Fig. 2). Viremia was not detected in any squirrels.
FIG. 2.

Seroprevalence and antibody titers of collected squirrels and minimum infection rate (MIR) of mosquitoes. (A) Bars show the percentage of seropositive individuals among squirrels collected by month. The black line represents the monthly mosquito MIR. (B) Dots represent each individual squirrel and its age class (adult/juvenile). WNV, West Nile virus; PRNT, plaque reduction neutralization test.
Mosquito collection
We collected a total of 4276 female mosquitoes, of which the majority (n=4131; 96.5%) were Culex (Cx.) spp., which were grouped into 287 pools. The remaining 146 specimens belonged to other genera and were grouped into 45 pools (unpublished data). In June, we collected the highest number of female mosquitoes per gravid trap (Fig. 1), and the number of collected mosquitoes dropped significantly (Mann–Whitney–Wilcoxon test, p<0.05) in July, and increased slightly in August and September (Fig. 1). The lowest number of collected mosquitoes per gravid trap was recorded in October (Fig. 1).
WNV was only detected in pools containing Culex spp. The WNV MIR for the entire study area was 5.8 (95% CI 3.7; 8.6) and ranged from 2.7 (95% CI 0.8; 6.2) to 9.8 (95% CI 0.2; 53.4) across sites. Mosquito infection peaked in August, and WNV was not detected in pools collected during September or October (Fig. 2). The lower WNV MIR recorded during July was not significantly different from June and August (Fisher LSD test, p=0.23). No significant difference in mosquito MIRs was observed between the sites (Fisher LSD test, p=0.34).
Model results
Two models were selected as the best candidates (Table 2). The variables included in these two best models were the monthly mosquito MIR (Σω=0.70) and squirrel age (Σω=0.38). The monthly MIR was significant and positively associated with squirrel seropositivity. Young squirrels had a lower probability of showing seropositivity than adults, but this difference was not significant. Antibody titers in September and October were significantly lower than in August according to the CLMM (Table 3). Gender and age were not correlated with antibody titer.
Table 2.
Generalized Linear Model (GLM) Models a
| Model | Intercept | Site | Age | Sex | Density | Monthly MIR | QIC | ΔQIC | ωi |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1.03* | — | — | — | — | 6.51×10–3* | 71.2 | 0 | 0.30 |
| 2 | −0.83* | — | −0.87 | — | — | 6.66×10–3* | 72.2 | 0.92 | 0.19 |
| 3 | −0.93* | — | — | −0.01 | — | 6.51×10−3* | 73.5 | 2.24 | 0.10 |
| 4 | −0.86* | — | −0.88 | 0.05 | −0.25 | 6.61×10−3* | 73.8 | 2.57 | 0.08 |
| 5 | −0.29 | — | −0.65 | — | — | — | 74.3 | 3.08 | 0.07 |
| 6 | −0.24 | −0.03 | — | — | 0.05 | 6.21×10−3* | 74.5 | 3.23 | 0.06 |
| Σωi | — | 0.14 | 0.38 | 0.24 | 0.09 | 0.70 |
The Σωi for each variable is included.
p<0.05.
Table 3.
Estimates of Parameters Based on the Cumulative Link Mixed Model (CLMM)
| Parameter | Effect (95% CI) |
|---|---|
| Sex (ref: male) | 0.12 (−0.91; 1.16) |
| Age (ref: juvenile) | −0.143 (−1.73; 1.42) |
| Month (ref: August): | |
| June | −1.21 (−2.68; 2.59) |
| September | −2.17 (−3.71; −0.62)** |
| October | −1.37 (−2.75; −0.01)* |
August was chosen as the reference month because it had the highest seroprevalence.
p<0.05.
p<0.01.
CI, confidence interval.
Discussion
Several studies have shown that spillover of WNV from the mosquito–bird cycle can be detected in many mammal species, but its extent and impact have been studied in depth only for humans. Previous studies reporting WNV seroprevalence in wild mammal communities did not address seasonality of virus spillover. In our study, we collected data on a fine temporal and spatial resolution, which allowed us to quantify monthly spatial and temporal spillover dynamics for a squirrel population living in a WNV hot spot in Atlanta.
Our findings point to WNV spillover into squirrels living in Grant Park (overall seroprevalence of 37.5%). This is similar to the seroprevalence levels recorded in other populations of S. carolinensis in the eastern and midwestern United States, including New York (Kramer and Bernard 2001, Marfin et al. 2001, Root et al. 2005), Illinois (Heinz-Taheny et al. 2004), Pennsylvania (Root et al. 2005), Louisiana (Dietrich et al. 2005), Maryland, and Washington DC (Gómez et al. 2008a).
The highest proportion of seropositive squirrels was reported in Grant Park in August, when the WNV MIR in Culex spp. mosquitoes and seroprevalence in birds captured in the study area also peaked in both 2010 and in 2011 (unpublished data). Thus, the high seroprevalence of WNV in squirrels in Grant Park corresponded to the period of maximum WNV transmission between vectors and avian reservoirs.
Spillover to squirrel populations may be facilitated by them sharing their habitat with avian reservoirs. Moreover, Cx. quinquefasciatus is one of the most abundant host-seeking mosquito species in canopy habitats (Savage et al. 2008), and a high prevalence of WNV infection was reported in mosquitoes collected in tree canopies (Anderson et al. 2004). This co-habitation contributes not only to the mosquito–bird WNV enzootic cycle, but, because Culex spp. females are opportunistic and also feed on several mammalian species (Apperson et al. 2002, Molaei et al. 2006, Hamer et al. 2008), it could explain the high seroprevalence in squirrels recorded here and in other studies.
The antibody titers of specimens collected in fall were significantly lower than in summer. This was consistent with absence of positive mosquito pools from this period. We also reported a low squirrel seroprevalence (no positive specimens) and mosquito MIR in July. In our study year, during July, 2012, we recorded the highest level of rainfall (8.2 cm) with 15 days of rain, which may have reduced the contact rate between vectors and hosts, limiting transmission. High levels of precipitation within a short period negatively impact survival of mosquito larvae (e.g., by flushing away egg rafts and larvae, and diluting nutrients) (Koenraadt and Harrington 2008) and may also reduce female host-seeking activity (Roiz et al. 2010), resulting in a negative impact on WNV circulation (Jones et al. 2012, Crowder et al. 2013). Our sample size in July was not sufficient to test the recorded reduction in seroprevalence for significance, but our data provide further indication that infection levels in squirrels may be associated with corresponding infection levels in vectors.
The correspondence between seasonality and level of seroprevalence of squirrels and birds collected in Grant Park could be linked to feeding behavior of mosquitoes circulating in the area. As shown by Kilpatrick et al. (2006), WNV spillover is driven by mosquito feeding preference, which determines contact rates between host and vectors. Our findings highlight the feeding of Atlanta mosquito vectors on mammals, a likely route for human spillover.
Seroprevalence in squirrels collected in our study may also be linked partially to nonvector transmission routes. It has been shown that WNV can be transmitted by oral inoculation or contact in birds (Komar et al. 2003, Bowen and Nemeth 2007). Although the diet of eastern gray squirrels consists primarily of nuts, this species is opportunistic and sometimes preys on nestling and adult passerine birds (Callahan 1993), a behavior that becomes more frequent during summer. Several studies have reported presence of virus in samples of saliva, feces, urine, and skin from infected tree squirrels, which can persist for weeks (Root et al. 2005, 2006, Padgett et al. 2007, Gómez et al. 2008b, Tiawsirisup et al. 2010), suggesting that some squirrels may be infected by contact with infected individuals that are shedding virus (e.g., through grooming, territorial fighting, communal nests).
Model selection showed that age was an important factor predicting seropositivity. Juvenile squirrels are less likely to be seropositive compared with adults. Although this result was not significant, it highlights that seroprevalence in young individuals is affected by the reduced exposure to the virus compared with adults. Low contact with virus of juveniles has been shown in eastern gray squirrels (Gómez et al. 2008a).
We did not detect significant differences in seroprevalence among squirrels collected in different sites, although the distance between trapping locations of our study was higher than the small home range (usually <5 ha) (Don 1983) of squirrels in urban areas, where food availability is high. Furthermore, the distance between trap locations was smaller than the home range of primary and secondary vectors for WNV (Brust 1980, Hamer et al. 2014), indicating that mosquitoes can transmit the virus to the squirrel population of Grant Park without creating spatial heterogeneity. Indeed, evidence of spatial heterogeneity in seroprevalence of squirrels has been detected only when collection sites were distributed at distances greater than mosquito flight range (Gómez et al. 2008a).
In summary, our findings show that the seasonal pattern of squirrel exposure to WNV is similar to that one experienced by birds living in Grant Park. Given the evidence of WNV circulation in their population and their small home range compared to birds, and possibly even to mosquitoes, tree squirrels may serve to identify WNV spillover in mammals at local scale. Furthermore, seroprevalence in tree squirrels may also be used to detect urban areas with high risk for human spillover due to vector feeding behavior.
Acknowledgments
This research was funded by research support from Emory University to U.K. and to E.N.V.S. students. Plaque-reduction neutralization tests were performed using intramural funds provided by Iowa State University.
We thank the students of the Vector Ecology Lab at Emory University who participated in the squirrel trapping and census. We also thank Jamie Allen, Stewart Haddock, Nat Slaughter, Laura Straub, and the rest of the Inman Park Squirrel Census team for their technical support during planning of collections and the squirrel census.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson JF, Andreadis TG, Main AJ, Kline DL. Prevalence of West Nile virus in tree canopy–inhabiting Culex pipiens and associated mosquitoes. Am J Trop Med Hyg 2004; 71:112–119. [PubMed] [Google Scholar]
- Apperson CS, Harrison BA, Unnasch TR, Hassan HK, et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the borough of queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol 2002; 39:777–785. [DOI] [PubMed] [Google Scholar]
- Beaty BJ, Calisher CH, Shoppe RE. Arboviruses. Diagnostic Procedures for Viral and Rickettsial Diseases. Washington, DC: American Public Health Association, 1995. [Google Scholar]
- Blitvich BJ, Bowen RA, Marlenee NL, Hall RA, et al. Epitope-blocking enzyme-linked immunosorbent assays for detection of West Nile virus antibodies in domestic mammals. J Clin Microbiol 2003; 41:2676–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich BJ, Juarez LI, Tucker BJ, Rowley WA, et al. Antibodies to West Nile virus in raccoons and other wild peridomestic mammals in Iowa. J Wildl Dis 2009; 45:1163–1168. [DOI] [PubMed] [Google Scholar]
- Bowen RA, Nemeth NM. Experimental infections with West Nile virus. Curr Opin Infect Dis 2007; 20:293–297. [DOI] [PubMed] [Google Scholar]
- Brust RA. Dispersal behavior of adult Aedes sticticus and Aedes vexans (Diptera: Culicidae) in Manitoba. Can Entomol 1980; 112:31–42. [Google Scholar]
- Bunning ML, Bowen RA, Cropp CB, Sullivan KG, et al. Experimental infection of horses with West Nile virus. Emerg Infect Dis 2002; 8:380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer, 2002. [Google Scholar]
- Bustamante DM, Lord CC. Sources of error in the estimation of mosquito infection rates used to assess risk of arbovirus transmission. Am J Trop Med Hyg 2010; 82:1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 1989; 70(Pt 1):37–43. [DOI] [PubMed] [Google Scholar]
- Callahan JR. Squirrels as predators. West North Am Nat 1993; 53:137–144. [Google Scholar]
- Centers for Disease Control and Prevention. West Nile Virus Homepage. 2014. Available at http://www.cdc.gov/ncidod/dvbid/westnile/index.htm
- Conover WJ. Non-Parametric Statistics. New York: Wiley & Sons, 1998. [Google Scholar]
- Crowder DW, Dykstra EA, Brauner JM, Duffy A, et al. West Nile virus prevalence across landscapes is mediated by local effects of agriculture on vector and host communities. PloS One 2013; 8:e55006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich G, Montenieri JA, Panella NA, Langevin S, et al. Serologic evidence of West nile virus infection in free-ranging mammals, Slidell, Louisiana, 2002. Vector Borne Zoonotic Dis 2005; 5:288–292. [DOI] [PubMed] [Google Scholar]
- Don BAC. Home range characteristics and correlates in tree squirrels. Mammal Rev 1983; 13:123–132. [Google Scholar]
- Flyger VF. A comparison of methods for estimating squirrel populations. J Wildl Manag 1959; 23:220. [Google Scholar]
- Gibbs SEJ, Allison AB, Yabsley MJ, Mead DG, et al. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector Borne Zoonotic Dis 2006; 6:57–72. [DOI] [PubMed] [Google Scholar]
- Gómez A, Kilpatrick AM, Kramer LD, Dupuis AP 2nd, et al. Land use and West Nile virus seroprevalence in wild mammals. Emerg Infect Dis 2008a;14:962–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez A, Kramer LD, Dupuis AP, 2nd, Kilpatrick AM, et al. Experimental infection of eastern gray squirrels (Sciurus carolinensis) with West Nile virus. Am J Trop Med Hyg 2008b; 79:447–451. [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ, Kuno G, Markoff L. Flaviviruses. In: Fields Virology, 5th ed. Philadelphia, PA; Lippincott Williams and Wilkins, 2007. [Google Scholar]
- Gustafson EJ, VanDruff LW. Behavior of black and gray morphs of Sciurus carolinensisin an urban environment. Am Midl Nat 1990; 123:186. [Google Scholar]
- Hamer GL, Kitron UD, Brawn JD, Loss SR, et al. Culex pipiens (Diptera: Culicidae): A bridge vector of West Nile virus to humans. J Med Entomol 2008; 45:125–128. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Anderson TK, Donovan DJ, Brawn JD, et al. Dispersal of adult Culex mosquitoes in an urban West Nile virus hotspot: A mark-capture study incorporating stable isotope enrichment of natural larval habitats. PLoS Negl Trop Dis 2014; 8:e2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein EW. Demonstration of line transect methodologies to estimate urban gray squirrel density. Environ Manage 1997; 21:943–947. [DOI] [PubMed] [Google Scholar]
- Heinz-Taheny KM, Andrews JJ, Kinsel MJ, Pessier AP, et al. West Nile virus infection in free-ranging squirrels in Illinois. J Vet Diagn Invest 2004; 16:186–190. [DOI] [PubMed] [Google Scholar]
- Jones CE, Lounibos LP, Marra PP, Kilpatrick AM. Rainfall influences survival of Culex pipiens (Diptera: Culicidae) in a residential neighborhood in the mid-Atlantic United States. J Med Entomol 2012; 49:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, et al. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol 2006; 4:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenraadt CJM, Harrington LC. Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae). J Med Entomol 2008; 45:28–35. [DOI] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis 2003; 9:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski JL. Sciurus carolinensis. Mamm Species 1994: 1–9. [Google Scholar]
- Kramer LD, Bernard KA. West Nile virus infection in birds and mammals. Ann NY Acad Sci 2001; 951:84–93. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol 2008; 53:61–81. [DOI] [PubMed] [Google Scholar]
- Levine RS, Mead DG, Kitron UD. Limited spillover to humans from West Nile virus viremic birds in Atlanta, Georgia. Vector Borne Zoonotic Dis 2013; 13:812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BR, McMullen AR, Swetnam DM, Barrett ADT. Molecular epidemiology and evolution of West Nile virus in North America. Int J Environ Res Public Health 2013; 10:5111–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manski DA, VanDruff LW, Flyger V. Activities of Gray Squirrels and People in a Downtown Washington, D.C. Park: Management implications. Wildlife Management Institute; 1981. [Google Scholar]
- Marfin AA, Petersen LR, Eidson M, Miller J; ArboNET Cooperative Surveillance Group. Widespread West Nile virus activity, eastern United States, 2000. Emerg Infect Dis 2001; 7:730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Anderson JF, et al. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis 2006; 12:468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett KA, Reisen WK, Kahl-Purcell N, Fang Y, et al. West Nile virus infection in tree squirrels (Rodentia: Sciuridae) in California, 2004–2005. Am J Trop Med Hyg 2007; 76:810–813. [PMC free article] [PubMed] [Google Scholar]
- Parker TS, Nilon CH. Gray squirrel density, habitat suitability, and behavior in urban parks. Urban Ecosyst 2008; 11:243–255. [Google Scholar]
- Petersen LR, Brault AC, Nasci RS. West Nile virus: Review of the literature. JAMA 2013; 310:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt KB, Tucker BJ, Halbur PG, Tiawsirisup S, et al. West Nile virus viremia in eastern chipmunks (Tamias striatus) sufficient for infecting different mosquitoes. Emerg Infect Dis 2007; 13:831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratterree MS, Gutierrez RA, Travassos da Rosa APA, Dille BJ, et al. Experimental infection of rhesus macaques with West Nile virus: level and duration of viremia and kinetics of the antibody response after infection. J Infect Dis 2004; 189:669–676. [DOI] [PubMed] [Google Scholar]
- Read RW, Rodriguez DB, Summers BA. West Nile virus encephalitis in a dog. Vet Pathol 2005; 42:219–222. [DOI] [PubMed] [Google Scholar]
- Reiter P, Jakob WL, Francy DB, Mullenix JB. Evaluation of the CDC gravid trap for the surveillance of St. Louis encephalitis vectors in Memphis, Tennessee. J Am Mosq Control Assoc 1986; 2:209–211. [PubMed] [Google Scholar]
- Roiz D, Rosà R, Arnoldi D, Rizzoli A. Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector Borne Zoonotic Dis 2010; 10:811–816. [DOI] [PubMed] [Google Scholar]
- Root JJ, Hall JS, McLean RG, Marlenee NL, et al. Serologic evidence of exposure of wild mammals to flaviviruses in the central and eastern United States. Am J Trop Med Hyg 2005; 72:622–630. [PubMed] [Google Scholar]
- Root JJ, Oesterle PT, Nemeth NM, Klenk K, et al. Experimental infection of fox squirrels (Sciurus niger) with West Nile virus. Am J Trop Med Hyg 2006; 75:697–701. [PubMed] [Google Scholar]
- Ruiz MO, Tedesco C, McTighe TJ, Austin C, et al. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int J Health Geogr 2004; 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Walker ED, Foster ES, Haramis LD, et al. Association of West Nile virus illness and urban landscapes in Chicago and Detroit. Int J Health Geogr 2007; 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM, Anderson M, Gordon E, McMillen L, et al. Host-seeking heights, host-seeking activity patterns, and West Nile virus infection rates for members of the Culex pipiens complex at different habitat types within the hybrid zone, Shelby County, TN, 2002 (Diptera: Culicidae). J Med Entomol 2008; 45:276–288. [DOI] [PubMed] [Google Scholar]
- Slaff M, Apperson CS. A Key to the Mosquitoes of North Carolina and the Mid-Atlantic States. Raleigh, NC: North Carolina State University, 1989. [Google Scholar]
- Steele MA, Koprowski JL. North American Tree Squirrels. Smithsonian Books, 2003. [Google Scholar]
- Tesh RB, Siirin M, Guzman H, Travassos da Rosa APA, et al. Persistent West Nile virus infection in the golden hamster: Studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis 2005; 192:287–295. [DOI] [PubMed] [Google Scholar]
- Tiawsirisup S, Platt KB, Tucker BJ, Rowley WA. Eastern cottontail rabbits (Sylvilagus floridanus) develop West Nile virus viremias sufficient for infecting select mosquito species. Vector Borne Zoonotic Dis 2005; 5:342–350. [DOI] [PubMed] [Google Scholar]
- Tiawsirisup S, Blitvich BJ, Tucker BJ, Halbur PG, et al. Susceptibility of fox squirrels (Sciurus niger) to West Nile virus by oral exposure. Vector Borne Zoonotic Dis 2010; 10:207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Vanden Eng JL, Kelly R, Mead DG, et al. The risk of West Nile virus infection is associated with combined sewer overflow streams in urban Atlanta, Georgia, USA. Environ Health Perspect 2010; 118:1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker JO, Hamilton WJ. Mammals of the Eastern United States. Ithaca, NY: Cornell University Press, 1998. [Google Scholar]



