ABSTRACT
Background
Hydration status plays a critical role in modulating oxidative stress during exercise, which can influence physical performance and recovery. While the acute effects of hydration on exercise-induced oxidative stress are well-documented, the long-term impact of chronic water intake remains poorly understood. Therefore, this study aimed to investigate the relationship between chronic low water intake and exercise-induced oxidative stress, as well as changes in the phenotypic composition of peripheral immune cells.
Methods
We assessed the usual plain water intake of the participants (n = 26; 19–29 years) using a questionnaire and classified them as habitually high-water drinkers (HIGH; n = 16; mean plain water intake = 1.22 ± 0.19 L/d) or low-water drinkers (LOW; n = 10; 0.41 ± 0.10 L/d). We conducted a maximal graded exercise test and investigated whether the extent of exercise-induced oxidative stress and immunological changes differed depending on the habitual water intake. Free radical production before and after the exercise test was assessed using serum concentrations of reactive oxygen metabolites (ROMs). The antioxidant capacity to eliminate free radicals was assessed using the serum biological antioxidant potential (BAP). We profiled peripheral blood mononuclear cells (PBMCs; CD4+, CD8+, CD20+, CD14+, CD11b+, and Annexin V+) using fluorescence-activated cell sorting.
Results
At baseline, the LOW group showed higher concentrations of serum ROMs than the HIGH group (p = 0.033). In a maximal graded exercise test, both groups showed comparable performance parameters including peak heart rate, VO2max, and exercise duration. However, the biochemical responses differed markedly: serum BAP significantly increased in the HIGH group but remained unchanged in the LOW group (p = 0.030). Furthermore, the LOW group showed a larger increase in PBMC apoptosis than the HIGH group, both in absolute cell number and percentage (p = 0.031 and p = 0.034, respectively). The LOW group also showed differential PBMC subset frequencies, with greater CD11b+ cell recruitment (p = 0.015) and less pronounced CD14+ cell reduction compared to the HIGH group (p = 0.050).
Conclusion
Habitual low water intake was associated with elevated concentrations of free radical by-products in the resting state and impaired antioxidant capacity during acute exercise stress. In response to exercise stress, impaired redox balance in low-water drinkers resulted in accelerated cellular damage and increased myeloid cell recruitment. These findings highlight the importance of maintaining adequate habitual water intake to cope with exercise-induced oxidative stress and prevent excessive cellular stress responses.
Clinical Trial Registration
Registered at the Clinical Research Information Service (CRIS) on 12 April 2019 (Registry No. KCT0003763).
KEYWORDS: Water intake, redox imbalance, oxidative stress, biological antioxidant potential, apoptosis, acute aerobic exercise
1. Introduction
Water is an essential nutrient that constitutes 50–70% of total body weight in healthy adults [1]. Hydration status, defined as the dynamic balance between water intake and water loss, is critical for biological processes including circulation, substance transport, and cellular metabolism [2]. Given these fundamental roles, maintaining adequate water intake is essential for optimal health and physiological function [3]. Adequate intake guidelines, based on median consumption values from national surveys, are widely employed as reference standards for evaluating individual water intake adequacy in research and public health [4,5]. Population studies using these guidelines have consistently reported that inadequate water consumption is prevalent among adults [6,7].
Body water homeostasis is maintained through complex physiological mechanisms that compensate for variations in water intake and loss [8]. This regulation prevents fluid deficits even when water consumption is chronically suboptimal, allowing individuals to avoid obvious signs of dehydration [9]. However, the absence of clinical dehydration symptoms does not necessarily indicate optimal physiological function [9]. Moreover, under metabolic stress conditions such as acute exercise, biological differences between individuals with habitually adequate and inadequate water intake may become more evident [10]. Nevertheless, research examining the relationship between chronic low water intake and underlying biochemical and cellular responses during exercise remains limited.
Oxidative stress refers to a state in which redox signaling and control are disturbed, resulting in undesirable oxidation of cellular components, such as lipids, proteins, and DNA [11]. Acute exercise dramatically increases oxidative stress through enhanced production of reactive oxygen species (ROS) [12]. Adequate hydration has been shown to protect against oxidative stress during exercise by maintaining cellular antioxidant capacity and reducing oxidative damage [13]. However, most research examining the relationship between hydration and oxidative stress has focused on acute dehydration, induced by short-term restriction of fluid intake during heat stress or strenuous activities [14,15]. While chronic low water intake is more prevalent in daily life, its relationship with oxidative stress during both rest and exercise remains poorly understood.
Therefore, we aimed to investigate the association between chronic water intake and oxidative stress by comparing habitual low- and high-water drinkers. Furthermore, we explored exercise-induced changes in the phenotypic composition of peripheral immune cells to determine how habitual water consumption patterns are associated with underlying cellular responses under acute oxidative stress. This investigation may provide valuable insights into hydration strategies for mitigating exercise-induced cellular stress and damage.
2. Methods
2.1. Study design and participants
As shown in Figure 1, this study followed a cross-sectional design to compare physiological and molecular responses to maximal graded exercise based on different habitual water intake levels. Participants were categorized into two groups according to their adherence to the Dietary Reference Intakes for plain water: high-water drinkers and low-water drinkers (Figure 1).
Figure 1.

Experimental design.
Participants were categorized into two groups according to their adherence to the Dietary Reference Intakes for plain water: high-water drinkers (HIGH) and low-water drinkers (LOW). The maximal graded exercise test was performed using a treadmill ergometer and the Bruce protocol to induce a standardized and consistent level of exertion across all participants. The exercise test was terminated after participants had reached the point of complete exhaustion. Blood samples were collected at rest (baseline; pre-exercise) and immediately after treadmill exercise (post-exercise) to measure oxidative stress markers and analyze peripheral blood mononuclear cell (PBMC) populations.
Study participants were recruited from a previous randomized controlled trial conducted in August 2016 through advertisements at Seoul National University and nearby neighborhoods [16]. Men and women aged 19–29 years with no medical history of acute or chronic diseases were eligible for inclusion. The exclusion criteria were designed to minimize inter-individual variation in baseline oxidative stress levels as follows: regular use of antioxidant supplements within the last three months; current or past smoking; alcohol consumption more than two days per week; and recent participation in vigorous exercise. Additionally, considering the diverse nutritional compositions and potential physiological effects of beverages other than plain water, participants were excluded if they habitually consumed beverages such as soft drinks, milk, juice, coffee, and tea exceeding 0.5 L per day. Of the 102 participants, 74 were excluded based on these criteria. Thus, 28 participants were eligible for the current study.
This study was performed at the Department of Food and Nutrition and Department of Physical Education at Seoul National University. The study was approved by the Institutional Review Board of Seoul National University (Approval number: 1606/001–012) and retrospectively registered at the Clinical Research Information Service on 12 April 2019 (Registry number: KCT0003763). All procedures were performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from all the participants prior to their inclusion in the study.
2.2. Laboratory assessment
Participants visited the laboratory between 0700 and 1000 hours after a 12-hour fast, having avoided alcohol consumption and strenuous physical activities on the previous day. During the 12-hour fasting period, participants were allowed to consume plain water according to their usual patterns until 2 hours before the laboratory visit, after which all fluid intake was restricted. Participants’ height (cm) and weight (kg) were measured using a digital scale (BSM330; InBody Co., Ltd., Seoul, Korea), with precision to the nearest 0.1 cm and 0.1 kg, respectively. Body mass index (BMI; kg/m2), total body water (L), lean body mass (kg), body fat mass (kg), body fat percentage (%), and waist-hip ratio were measured using a bioimpedance analyzer (InBody770; InBody Co., Ltd., Seoul, Korea). Physical activity levels were assessed using the International Physical Activity Questionnaire [17]. Habitual alcohol consumption was assessed using a questionnaire validated for young adults [18].
2.2.1. Assessment of usual intake of drinking water
The usual intake of drinking water was defined as the amount of regularly consumed plain water. The plain water intake of the participants was investigated using questionnaires [19]. Other beverages, including soft drinks, milk, juices, coffee, and tea, were excluded from the plain water intake calculation. We asked about the types of plain water and beverages consumed per day, number of intakes, and amount of intake per serving. The Dietary Reference Intakes for Koreans suggest an adequate plain water intake of ≥981 mL for men and ≥709 mL for women in the 19–29 age group [20]. Based on this, participants whose plain water intake met or exceeded the recommended adequate intake were classified as high-water drinkers (HIGH), while those whose intake fell below the recommendation were classified as low-water drinkers (LOW).
2.2.2. Maximal graded exercise test
The participants completed a physical activity readiness questionnaire (PAR-Q) to determine whether they had any problems or hazards of partaking in an exercise test [21]. Based on their responses to the PAR-Q, we confirmed that all participants were eligible to participate in the acute aerobic exercise test. The maximal graded exercise test was performed using a treadmill ergometer (T150; COSMED, Rome, Italy) equipped with a metabolic cart (QUARK; COSMED, Rome, Italy) and the Bruce protocol to induce a standardized and consistent level of exertion across all participants [22]. The test began at 1.7 mph at a 10% grade, and both the speed and grade gradually increased every 3 minutes (i.e. 2.5 mph at 12%, 3.4 mph at 14%, 4.2 mph at 16%, etc.). The participants’ heart rate (beats per minute; bpm) and VO2 (mL/min) were monitored in real-time by well-trained supervisors. Participants reported the rating of perceived exertion based on the Modified Borg Rating scale every three minutes, and the exercise test was terminated after the participant had reached the point of complete exhaustion [23]. All tests were conducted for 14–22 min at a temperature of 21–23 °C and humidity of 50–60%.
2.2.3. Blood analyses
Blood samples were collected twice: at a resting state (baseline; pre-exercise) and immediately after treadmill exercise (post-exercise) (Figure 1). Fasting venous blood samples from the antecubital fossa were collected in 8-mL serum separator tubes (BD Biosciences, Franklin Lakes, NJ, USA) and 4-mL EDTA-containing tubes (BD Biosciences, Franklin Lakes, NJ, USA). Serum preparation was performed via centrifugation, and the separated serum samples were aliquoted into 1.5-mL tubes (Eppendorf, Hamburg, Germany) and immediately stored at −80 °C.
Serum samples were used to measure oxidative damage and antioxidant capacity. Serum concentrations of reactive oxygen metabolites (ROMs) were assessed using hydroperoxide concentrations (Diacron Reactive Oxygen Metabolites Kit; Diacron Srl., Grosseto, Italy). The concentration of ROMs was expressed as Carratelli units (U.CARR) where 1 U.CARR corresponds to 0.08 mg/dL of hydrogen peroxide. Antioxidant capacity was determined using the biological antioxidant potential (BAP) test (BAP Kit; Diacron Srl., Grosseto, Italy), which measures the amount of reduced ferric ions proportional to the antioxidant capacity of the serum sample [24]. The measurements of the BAP test were expressed in µmol/L of reduced iron. All serum biochemistry tests were performed in duplicate according to manufacturer’s instructions. For all assays, intra- and inter-assay coefficients of variation were < 10%.
2.2.4. Fluorescence-activated cell sorting
Peripheral blood mononuclear cells (PBMCs) were isolated from 4 mL of whole blood collected in EDTA tubes by density-gradient centrifugation using Ficoll-Paque PLUS density gradient media (GE Healthcare, Songdo, Korea). PBMCs were stained with Alexa Fluor 488-conjugated anti-human CD4 (OKT4; eBioscience, San Diego, CA, USA), PE-conjugated anti-human CD8 (3B5; eBioscience), APC-Cy7-conjugated anti-human CD20 (B-Ly-1; eBioscience), APC-conjugated anti-human CD14 (61D3; eBioscience), and APC-Cy7-conjugated anti-human CD11b (ICRF44; BD Biosciences, San Jose, CA, USA) antibodies in FACS buffer (0.1% bovine calf serum and 0.05% sodium azide in 1× PBS) at 4 °C for 30 min. To assess apoptosis in PBMCs, Annexin V staining was performed using PE-conjugated anti-annexin V antibody (eBioscience) in annexin V binding buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, and 2.5 mM CaCl2) at room temperature for 15 min. DAPI (4′,6-diamidino-2-phenylindole; Sigma-Aldrich, St. Louis, MO, USA) staining was used to discriminate between live and dead cells. Profiles of each population were analyzed using a FACS LSRFortessa flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) with FlowJo software (TreeStar, Ashland, OR, USA).
2.3. Statistical analysis
Of the 28 initial participants, 2 were unable to participate in the post-exercise blood collection because of dizziness. As a result, a total of 26 participants were included in the final statistical analysis. Low-water drinkers were statistically compared with high-water drinkers. Differences in baseline general characteristics were analyzed using an unpaired t-test or Mann–Whitney U test for continuous variables depending on normality, and Fisher’s exact test for categorical variables. A general linear model was used to compare baseline measures adjusting for sex, age (y), BMI (kg/m2), alcohol intake (g/week), and physical activity (sedentary, light, or moderate). A paired t-test or Wilcoxon signed-rank test was used for comparisons between baseline and post-exercise measures within each group. A linear mixed effect model for the measures was used to determine the interaction between exercise (pre- and post-exercise) and the water intake level (HIGH and LOW) while accounting for sex, age, BMI, alcohol intake, and physical activity, and the subject term was used as a random effect. For multiple comparisons in FACS measures, p-values were adjusted using the Benjamini-Hochberg false discovery rate method. All statistical analyses were performed using R v.4.1.1 for Macintosh. p ≤ 0.05 was considered statistically significant.
3. Results
3.1. Participants’ general characteristics and exercise performance
Table 1 presents the participants’ demographic and anthropometric characteristics, plain water and beverage intake, alcohol consumption, and physical activity levels. The HIGH group demonstrated significantly higher plain water intake (men, 1.28 ± 0.16 L/d; women, 1.15 ± 0.22 L/d; total, 1.22 ± 0.19 L/d) compared to the LOW group (men, 0.42 ± 0.08 L/d; women, 0.40 ± 0.14 L/d; total, 0.41 ± 0.10 L/d), representing an approximately threefold difference (p < 0.001; Table 1). The LOW group did not differ from the HIGH group in beverage intake (HIGH, 0.25 ± 0.16 L/d; LOW, 0.33 ± 0.07 L/d; p = 0.187; Table 1). There were no significant differences in sex ratio, age, BMI, and anthropometric characteristics, including total body water between the LOW and HIGH groups (all p > 0.05; Table 1). Additionally, alcohol intake and physical activity levels did not differ between the groups (all p > 0.05; Table 1). In the maximal graded exercise test, the LOW group did not differ from the HIGH group in exercise performance measures including peak heart rate, VO2max, and duration (all p > 0.05; Table 2).
Table 1.
General characteristics of the participants.
| HIGH (n = 16) | LOW (n = 10) | p | |
|---|---|---|---|
| Sex, n (%) | 16 | 10 | .466 |
| Men | 8 (50.0) | 6 (60.0) | |
| Women | 8 (50.0) | 4 (40.0) | |
| Age, y | 24.5 (1.9) | 25.3 (2.9) | .402 |
| Height, cm | 168.1 (8.4) | 169.0 (7.9) | .774 |
| Weight, kg | 64.0 (12.3) | 64.7 (9.2) | .883 |
| BMI, kg/m2 | 22.6 (2.6) | 22.5 (2.0) | .986 |
| Total body water, L | 35.7 (8.5) | 35.3 (8.2) | .896 |
| Lean body mass, kg | 48.8 (11.6) | 48.2 (11.2) | .894 |
| Body fat mass, kg | 15.2 (4.5) | 16.5 (4.0) | .468 |
| Body fat percentage, % | 24.1 (7.2) | 26.3 (8.2) | .486 |
| Waist-hip ratio | 0.83 (0.05) | 0.84 (0.05) | .663 |
| Plain water intake, L/d | 1.22 (0.19) | 0.41 (0.10) | < .001 |
| Men | 1.28 (0.16) | 0.42 (0.08) | < .001 |
| Women | 1.15 (0.22) | 0.40 (0.14) | < .001 |
| Beverage intake, L/d | 0.25 (0.16) | 0.33 (0.07) | .187 |
| Alcohol intake, g/week | 16.5 (28.9) | 9.9 (26.5) | .564 |
| Physical activity level, n (%) | .391 | ||
| Sedentary or light | 9 (56.3) | 7 (70) | |
| Moderate | 7 (43.7) | 3 (30) |
Values are mean (SD) or n (%).
p-values were determined using an unpaired t-test or Mann–Whitney U test for continuous variables depending on normality, and Fisher’s exact test for categorical variables.
HIGH, high-water drinkers; LOW, low-water drinkers.
Table 2.
Performance in the maximal graded exercise test.
| HIGH (n = 16) | LOW (n = 10) | p | |
|---|---|---|---|
| Peak heart rate, bpm | 197.5 (2.7) | 195.8 (2.6) | .356 |
| VO2max, mL/min/kg | 39.4 (3.7) | 37.6 (4.6) | .202 |
| Duration, min | 18.0 (1.8) | 17.4 (1.8) | .462 |
All values are presented as mean (SD).
p-values were determined using an unpaired t-test.
HIGH, high-water drinkers; LOW, low-water drinkers; bpm, beats per minute.
3.2. Reactive oxygen metabolites and biological antioxidant potential
We measured the amount of free radicals with serum concentrations of ROMs and determined the antioxidant capacity for eliminating free radicals with serum BAP. At baseline, the LOW group had higher concentrations of ROMs than the HIGH group (HIGH, 338.3 ± 54.3 U.CARR; LOW, 388.4 ± 42.1 U.CARR; p = 0.033; Table 3). For serum BAP, there was no significant difference between the LOW and HIGH groups at baseline (HIGH, 2325.6 ± 366.7 µmol/L; LOW, 2347.1 ± 355.0 µmol/L; p > 0.05; Table 3).
Table 3.
Serum concentrations of reactive oxygen metabolites and serum biological antioxidant potential.
| HIGH (n = 16) |
LOW (n = 10) |
LOW vs. HIGH |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-exercise | Change | Baseline | Post-exercise | Change | pa | pb | |
| ROMs, U.CARR | 338.3 (54.3) | 369.3 (48.8) | 31.0 (13.8)*** | 388.4 (42.1) | 410.3 (49.8) | 21.9 (28.7)* | 0.033 | 0.416 |
| BAP, µmol/L | 2325.6 (366.7) | 2487.4 (370.3) | 161.9 (200.3)** | 2347.1 (355.0) | 2332.6 (198.6) | −14.5 (305.5) | 0.892 | 0.030 |
All values are presented as mean (SD).
aA general linear model was used to determine differences in baseline measures adjusting for sex, age, BMI, alcohol intake, and physical activity.
bA linear mixed effect model for ROMs or BAP was used to determine the interaction between exercise and water intake while adjusting for sex, age, BMI, alcohol intake, and physical activity, with subject as a random effect.
Within-group comparisons between baseline and post-exercise measures were analyzed using a paired t-test. *p < 0.05, **p < 0.01, ***p < 0.001.
HIGH, high-water drinkers; LOW, low-water drinkers; ROMs, reactive oxygen metabolites; U.CARR, Carratelli Units; BAP, biological antioxidant potential.
We induced acute metabolic stress using a standardized exercise test and determined the immediate changes in the serum levels of ROMs and BAP. The single bout of treadmill exercise induced an increase in the concentrations of serum ROMs in both groups without a significant group difference in its change (HIGH, Δ = 31.0 ± 13.8 U.CARR; LOW, Δ = 21.9 ± 28.7 U.CARR; p > 0.05; Table 3). Conversely, the LOW and HIGH groups showed different patterns of change in serum BAP. In the HIGH group, BAP increased by 7% of the baseline value due to exercise stress, whereas the LOW group did not show an increase in BAP, resulting in a significant group-difference (HIGH, Δ = 161.9 ± 200.3 µmol/L; LOW, Δ = −14.5 ± 305.5 µmol/L; p = 0.030; Table 3).
3.3. PBMC apoptosis
The number and proportion of apoptotic cells are shown in Figure 2. At baseline, we did not observe a significant difference in apoptosis between the HIGH and LOW groups (all p > 0.05; Figure 2). A single bout of intense exercise induced a dramatic increase in apoptosis; the number of apoptotic cells doubled in the HIGH group (p < 0.05; Figure 2). Notably, the LOW group presented an increase of 177% in the number of apoptotic cells (p < 0.001; Figure 2), which was much greater than that of the HIGH group (p = 0.031; Figure 2). In addition, for the proportion of apoptotic cells in the total PBMCs, the HIGH group showed a decreasing trend, while the LOW group showed an increasing trend, resulting in a significant difference in the change (p = 0.034; Figure 2).
Figure 2.
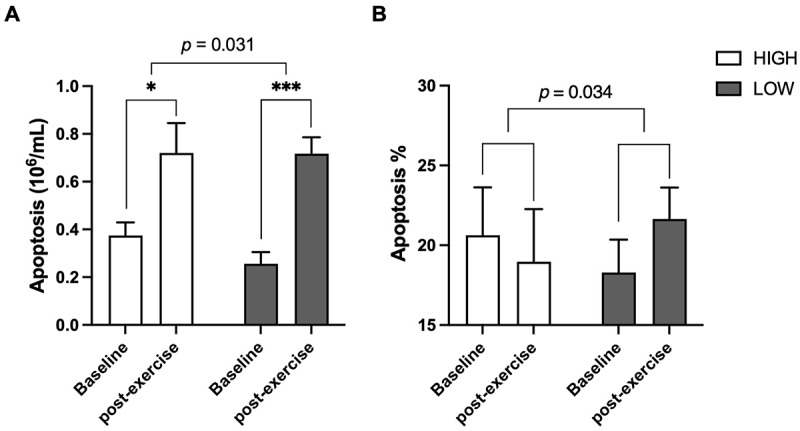
Apoptosis in peripheral blood mononuclear cells.
Low-water drinkers (LOW; n = 10) showed a greater increase in apoptosis compared to high-water drinkers (HIGH; n = 16). (A) Absolute number of apoptotic cells. (B) Percentage of apoptotic cells among total peripheral blood mononuclear cells. Within-group differences between baseline and post-exercise measures were determined using a paired t-test (*p < 0.05 and ***p < 0.001). A linear mixed effects model for apoptosis was used to determine the interaction between exercise and water intake while adjusting for sex, age, BMI, alcohol intake, and physical activity, with subject as a random effect.
3.4. PBMC subset
We analyzed the profiles of helper T cells (CD4+), cytotoxic T cells (CD8+), B cells (CD20+), and myeloid cells (CD14+ and CD11b+) from PBMCs and presented their proportions in Table 4. At baseline, the HIGH and LOW groups did not show significant differences in the proportion of peripheral immune cell subsets (all p > 0.05; Table 4). Intense aerobic exercise immediately altered the frequency of subsets in the HIGH group: the proportions of CD4+, CD20+, and CD14+ cells significantly decreased (CD4+, Δ = −12.66 ± 4.52%; CD20+, Δ = −4.26 ± 2.42%; CD14+, Δ = −3.46 ± 4.70%; all p < 0.001), whereas that of CD11b+ cells significantly increased (Δ = 16.04 ± 6.88%; p < 0.001; Table 4). Notably, changes in peripheral myeloid cells were significantly different between the HIGH and LOW groups (Table 4). In response to exercise, the LOW group showed a smaller decrease in the frequency of CD14+ cells (Δ = −0.85 ± 1.64%) and a larger increase in CD11b+ cells (Δ = 25.58 ± 6.81%) compared with the HIGH group (p = 0.050 and p = 0.015, respectively; Table 4).
Table 4.
Frequency of peripheral blood mononuclear cell subsets.
| HIGH (n = 16) |
LOW (n = 10) |
LOW vs. HIGH |
||||||
|---|---|---|---|---|---|---|---|---|
| Proportion (%) | Baseline | Post-exercise | Change | Baseline | Post-exercise | Change | pa | pb |
| CD4+ | 32.76 (9.05) | 20.10 (7.01) | −12.66 (4.52)*** | 28.55 (14.11) | 18.26 (3.84) | −10.29 (13.56) | 0.383 | > 1.0 |
| CD8+ | 28.62 (6.92) | 30.10 (8.44) | 1.48 (4.33) | 24.11 (4.20) | 25.09 (6.27) | 0.98 (4.40) | 0.107 | > 1.0 |
| CD20+ | 11.02 (3.22) | 6.78 (1.98) | −4.26 (2.42)*** | 9.70 (4.45) | 4.51 (1.67) | −5.19 (3.43)** | 0.414 | 0.345 |
| CD14+ | 8.59 (10.03) | 5.14 (8.71) | −3.46 (4.70)*** | 3.88 (2.95) | 3.03 (1.91) | −0.85 (1.64) | 0.153 | 0.050 |
| CD11b+ | 38.46 (11.95) | 54.51 (10.80) | 16.04 (6.88)*** | 36.00 (6.12) | 61.59 (3.69) | 25.58 (6.81)*** | 0.593 | 0.015 |
All values are presented as mean (SD).
aA general linear model was used to determine differences in baseline measures adjusting for sex, age, BMI, alcohol intake, and physical activity.
bA linear mixed effect model for the percentage of each immune cell population was used to determine the interaction between exercise and water intake while adjusting for sex, age, BMI, alcohol intake, and physical activity, with subject as a random effect.
Within-group comparisons between baseline and post-exercise measures were analyzed using a paired t-test or Wilcoxon signed-rank test depending on normality. **p < 0.01, ***p < 0.001.
HIGH, high-water drinkers; LOW, low-water drinkers.
4. Discussion
To the best of our knowledge, this is the first study to report a relationship between usual water intake and oxidative stress markers and peripheral immune cell responses to acute exercise. In the current study, participants were classified as either high-water drinkers (HIGH) or low-water drinkers (LOW) according to their usual intake of water. The HIGH group had a regular intake of plain water of more than 1.2 L/d, while the LOW group drank less than 0.5 L/d. Even at rest without any physiological stress, the LOW group showed higher serum concentrations of ROMs than the HIGH group. In the maximal graded exercise test conducted to assess oxidative stress and immune responses, both groups showed similar exercise performance, with no significant differences in peak heart rate, VO2max, and duration. However, despite this functional similarity, the underlying physiological responses to acute exercise stress differed markedly between groups; the LOW group had attenuated antioxidant capacity and showed greater increases in PBMC apoptosis and myeloid cell recruitment compared with the HIGH group. These findings suggest that chronic low water intake compromises the body’s ability to cope with exercise-induced physiological stress.
It is well established that adequate hydration is critical for maintaining optimal physical performance and mitigating exercise-induced oxidative stress [25,26]. For example, Paik et al. reported that heat exposure-induced dehydration impaired exercise performance and increased DNA oxidative damage during treadmill exercise [14]. Likewise, Hillman et al. observed that intense cycling-induced dehydration significantly increased blood oxidized glutathione concentrations, whereas adequate hydration attenuated these increases in trained athletes [15]. Different from these findings where acute dehydration impaired performance and increased oxidative stress, our study found that individuals with chronically low water intake exhibited markedly elevated oxidative stress responses with no decline in exercise capacity. Importantly, these individuals did not show clinical dehydration signs at baseline with normal total body water content [27]. This pattern represents underhydration rather than dehydration, as dehydration status is typically characterized by reduced total body water, increased plasma osmolality, and thirst sensation [9]. These distinctions are particularly important because physiological adaptations to chronic insufficient water intake may maintain normal hydration and exercise capacity while masking significant underlying cellular stress. This may lead athletes to underestimate the need for chronic hydration optimization.
Our findings provide important insights into how chronic low water intake is associated with underlying adverse cellular responses during exercise. While exercise predictably increases ROS generation, the concurrent increase in antioxidant capacity serves as a protective mechanism against oxidative stress [13,28]. However, the LOW group exhibited significant ROS increases but failed to show antioxidant capacity improvements during acute exercise. These findings indicate that chronic low water intake may reduce the concentration or impair the activity of circulating antioxidants during metabolically demanding situations. Under these conditions, oxidative stress can overwhelm cellular repair mechanisms, leading to programmed cell death to eliminate cells with irreversible damage [29]. In our study, low-water drinkers showed a much more pronounced increase in apoptosis after exercise compared to high-water drinkers, suggesting greater susceptibility to severe cellular damage due to reduced antioxidant defense activity. In line with these observations, low water consumption was associated with greater mobilization of CD11b+ and CD14+ myeloid cells during exercise compared to high-water drinkers. Myeloid cells are recruited into the circulation to clear damaged cells via phagocytic mechanisms and mediate inflammatory responses, both of which involve ROS generation via NADPH oxidase [30,31]. Under conditions of impaired antioxidant capacity, excessive ROS production can cause additional tissue damage, which further activates myeloid cells. This perpetuates a feed-forward inflammatory cycle characterized by continued myeloid cell recruitment and enhanced ROS generation [32,33]. Collectively, our findings suggest the possibility that chronic adaptation to low water intake involves a physiological trade-off during acute stress, which comes at the expense of dynamic responsiveness of antioxidant systems. While these individuals maintain baseline water homeostasis and comparable exercise performance, this adaptive impairment in antioxidant systems may render them more susceptible to the detrimental oxidative and inflammatory cycles when exposed to metabolically demanding conditions.
Although immediate exercise performance was maintained, the compromised antioxidant responsiveness during acute exercise in low-water drinkers may have profound implications for long-term physiological resilience, injury susceptibility, and recovery outcomes among athletes and physically active individuals [34]. Elevated levels of ROS during exercise serve as a critical marker of muscle damage that can delay recovery [35]. Previous studies have demonstrated that exercise-induced ROS elevation is mediated by inflammatory signaling involving transcription factors such as nuclear factor κB, which can further impair recovery outcomes [36–38]. Consequently, the impaired antioxidant capacity and increased cellular damage may lead to prolonged recovery periods between training sessions or competitions, interfering with training adaptations and long-term performance improvements [34,39]. The coordinated pattern observed in low-water drinkers – increased oxidative stress, enhanced cellular damage, and greater inflammatory cell recruitment – strongly suggests that these individuals may experience impaired recovery and increased fatigue in the post-exercise period. Our findings highlight that hydration assessment in athletic populations should extend beyond traditional markers of acute dehydration to include chronic water intake patterns in a comprehensive evaluation. Moreover, evaluating both functional outcomes and underlying molecular responses is essential to fully understand the exercise-related physiological consequences of optimal hydration.
Several limitations of this study should be acknowledged. First, the cross-sectional design limits our ability to establish causal relationships between chronic water intake patterns and the observed physiological responses. Intervention studies are needed to determine whether increasing water intake in chronically low drinkers can normalize oxidative stress responses both at rest and during exercise. Second, we assessed only immediate post-exercise responses and did not examine recovery markers over extended periods, which would provide more direct evidence for the proposed implications on recovery and adaptation. Third, while we assessed recent physical activity levels and controlled for these in our analyses, we did not collect detailed information on long-term exercise training history or specific training modalities. Different training backgrounds may influence oxidative stress responses to acute exercise independent of hydration status, which should be considered in future studies.
Despite these limitations, this study provides valuable insights by demonstrating differential biochemical and cellular responses to acute exercise according to habitual water intake patterns, underscoring the importance of maintaining adequate water consumption habits beyond acute hydration status.
Acknowledgments
This work is dedicated to the memory of Professor Dong-Mi Shin, whose visionary ideas and unwavering support were instrumental in shaping this research.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT), Korea [No. RS-2024-00339159].
Authors’ contributions
Minju Sim: conceptualization, investigation, project administration, data curation, methodology, formal analysis, visualization, and writing – original draft; Wook Song and Eun Young Choi: methodology; Dong-Mi Shin: conceptualization, funding acquisition, methodology, resources, supervision; Chong-Su Kim: conceptualization, funding acquisition, supervision, and writing – review & editing. All authors read and approved the final version of the manuscript. Minju Sim and Chong-Su Kim have accessed and verified the underlying data. Chong-Su Kim had primary responsibility for final content.
Disclosure statement
No potential conflict of interest was reported by the authors.
Consent for publication
Not applicable. This manuscript does not include any identifiable personal data requiring specific consent for publication.
Data availability statement
The datasets analyzed in the present current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki. Ethics approval was obtained from the Institutional Review Board of Seoul National University (Approval Number: 1606/001–012). All participants provided written informed consent before enrollment, and their rights and privacy were fully protected throughout the study.
References
- 1.Watson PE, Watson ID, Batt RD.. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33(1):27–11. doi: 10.1093/ajcn/33.1.27 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong LE. Assessing hydration status: the elusive gold standard. J Am Coll Nutr. 2007;26(sup5):575S–584S. doi: 10.1080/07315724.2007.10719661 [DOI] [PubMed] [Google Scholar]
- 3.Jéquier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. 2010;64(2):115–123. doi: 10.1038/ejcn.2009.111 [DOI] [PubMed] [Google Scholar]
- 4.Gandy J. Water intake: validity of population assessment and recommendations. Eur J Nutr. 2015;54(S2):11–16. doi: 10.1007/s00394-015-0944-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on Dietary reference values for water. EFSA J. 2010;8(3). doi: 10.2903/j.efsa.2010.1459 [DOI] [Google Scholar]
- 6.Kim J, Yang YJ. Plain water intake of Korean adults according to life style, anthropometric and dietary characteristic: the Korea National Health and Nutrition examination surveys 2008–2010. Nutr Res Pract. 2014;8(5):580. doi: 10.4162/nrp.2014.8.5.580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drewnowski A, Rehm CD, Constant F. Water and beverage consumption among adults in the United States: cross-sectional study using data from NHANES 2005–2010. BMC Public Health. 2013;13(1). doi: 10.1186/1471-2458-13-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danziger J, Zeidel ML. Osmotic homeostasis. Clin J Am Soc Nephrol. 2015;10(5):852–862. doi: 10.2215/CJN.10741013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavouras SA. Hydration, dehydration, underhydration, optimal hydration: are we barking up the wrong tree? Eur J Nutr. 2019;58(2):471–473. doi: 10.1007/s00394-018-01889-z [DOI] [PubMed] [Google Scholar]
- 10.King MA, Clanton TL, Laitano O. Hyperthermia, dehydration, and osmotic stress: unconventional sources of exercise-induced reactive oxygen species. Am J Physiol Regul Intergr Comp Physiol. 2016;310(2):R105–R114. doi: 10.1152/ajpregu.00395.2015 [DOI] [PubMed] [Google Scholar]
- 11.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med. 2009;8(1). doi: 10.1186/1476-5918-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laitano O, Kalsi KK, Pearson J, et al. Effects of graded exercise-induced dehydration and rehydration on circulatory markers of oxidative stress across the resting and exercising human leg. Eur J Appl Physiol. 2012;112(5):1937–1944. doi: 10.1007/s00421-011-2170-2 [DOI] [PubMed] [Google Scholar]
- 14.Paik I-Y, Jeong M-H, Jin H-E, et al. Fluid replacement following dehydration reduces oxidative stress during recovery. Biochem Biophys Res Commun. 2009;383(1):103–107. doi: 10.1016/j.bbrc.2009.03.135 [DOI] [PubMed] [Google Scholar]
- 15.Hillman AR, Vince RV, Taylor L, et al. Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress. Appl Physiol Nutr Metab. 2011;36(5):698–706. doi: 10.1139/h11-080 [DOI] [PubMed] [Google Scholar]
- 16.Sim M, Kim C-S, Shon W-J, et al. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: a randomized, double-blind, controlled trial. Sci Rep. 2020;10(1). doi: 10.1038/s41598-020-68930-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee PH, Macfarlane DJ, Lam T, et al. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8(1). doi: 10.1186/1479-5868-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehrabian A, Russell JA. A questionnaire measure of habitual alcohol use. Psychol Rep. 1978;43(3):803–806. doi: 10.2466/pr0.1978.43.3.803 [DOI] [PubMed] [Google Scholar]
- 19.Ji K, Kim Y, Choi K. Water intake rate among the general Korean population. Sci Total Environ. 2010;408(4):734–739. doi: 10.1016/j.scitotenv.2009.10.076 [DOI] [PubMed] [Google Scholar]
- 20.Kwon O, Kim H, Kim J, et al. The development of the 2020 Dietary reference Intakes for Korean population: lessons and challenges. J Nutr Health. 2021;54(5):425. doi: 10.4163/jnh.2021.54.5.425 [DOI] [Google Scholar]
- 21.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q). Can J Sport Sci. 1992;17(4):338–345. [PubMed] [Google Scholar]
- 22.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85(4):546–562. doi: 10.1016/0002-8703(73)90502-4 [DOI] [PubMed] [Google Scholar]
- 23.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 24.Matsuura T, Kaneko H, Takayama K, et al. Diacron reactive oxygen metabolites and biological antioxidant potential tests for patients with age-related macular degeneration. BMC Ophthalmol. 2020;20(1). doi: 10.1186/s12886-020-01334-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCartney D, Desbrow B, Irwin C. The effect of fluid intake following dehydration on subsequent athletic and cognitive performance: a systematic review and meta-analysis. Sports Med Open. 2017;3(1). doi: 10.1186/s40798-017-0079-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goulet ED. Dehydration and endurance performance in competitive athletes. Nutr Rev. 2012;70:S132–S136. doi: 10.1111/j.1753-4887.2012.00530.x [DOI] [PubMed] [Google Scholar]
- 27.Shanholtzer BA, Patterson SM. Use of bioelectrical impedance in hydration status assessment: reliability of a new tool in psychophysiology research. Int J Psychophysiol. 2003;49(3):217–226. doi: 10.1016/S0167-8760(03)00143-0 [DOI] [PubMed] [Google Scholar]
- 28.Parker L, McGuckin TA, Leicht AS. Influence of exercise intensity on systemic oxidative stress and antioxidant capacity. Clin Physiol Funct Imaging. 2014;34(5):377–383. doi: 10.1111/cpf.12108 [DOI] [PubMed] [Google Scholar]
- 29.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16(6):663–669. doi: 10.1016/j.ceb.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 30.Canton M, Sánchez-Rodríguez R, Spera I, et al. Reactive oxygen species in macrophages: sources and targets. Front Immunol. 2021;12:12. doi: 10.3389/fimmu.2021.734229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herb M, Schramm M. Functions of ROS in macrophages and antimicrobial immunity. Antioxidants. 2021;10(2):313. doi: 10.3390/antiox10020313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen M-F, Kuan F-C, Yen T-C, et al. IL-6-stimulated CD11b+CD14+HLA-DR− myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5(18):8716–8728. doi: 10.18632/oncotarget.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dmytriv TR, Duve KV, Storey KB, et al. Vicious cycle of oxidative stress and neuroinflammation in pathophysiology of chronic vascular encephalopathy. Front Physiol. 2024;15:15. doi: 10.3389/fphys.2024.1443604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bessa AL, Oliveira VN, Agostini G, et al. Exercise intensity and recovery: biomarkers of injury, inflammation, and oxidative stress. J Strength Cond Res. 2016;30(2):311–319. doi: 10.1519/JSC.0b013e31828f1ee9 [DOI] [PubMed] [Google Scholar]
- 36.Slattery K, Bentley D, Coutts AJ. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. 2015;45(4):453–471. doi: 10.1007/s40279-014-0282-7 [DOI] [PubMed] [Google Scholar]
- 37.Batista MCC, Dos Santos MAP. Impact of hydration on exercise performance and physiological responses. Curr Nutr Food Sci. 2020;16(9):1346–1352. doi: 10.2174/1573401316666200309113907 [DOI] [Google Scholar]
- 38.Fernández-Lázaro D, Mielgo-Ayuso J, Seco Calvo J, et al. Modulation of exercise-induced muscle damage, inflammation, and oxidative markers by curcumin supplementation in a physically active population: a systematic Review. Nutrients. 2020;12(2):501. doi: 10.3390/nu12020501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powers SK, Deminice R, Ozdemir M, et al. Exercise-induced oxidative stress: friend or foe? J Sport Health Sci. 2020;9(5):415–425. doi: 10.1016/j.jshs.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in the present current study are available from the corresponding author upon reasonable request.


