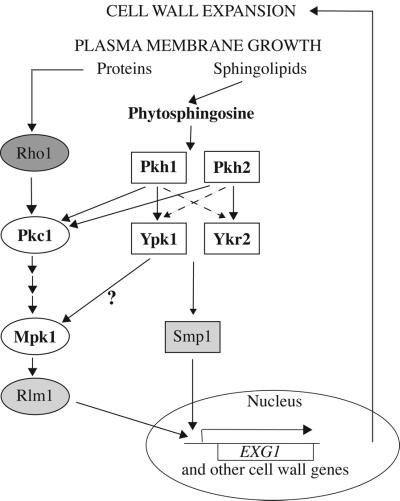
Figure 9.
Pkh1-Ypk1 and Pkh2-Ykr2 cascades and cell wall integrity signaling. For proper cell enlargement, plasma membrane growth needs to be accommodated by expansion of the cell wall. This coordinated coupling is achieved through at least two pathways. As shown previously, one route for monitoring plasma membrane growth is via the insertion of transmembrane proteins that affect the state of activation of the small GTPase, Rho1. Rho1, in turn, stimulates the protein kinase, Pkc1, and the downstream MAP kinase kinase cascade (Bck1 → Mkk1 and Mkk2 → Mpk1). The Mpk1 MAP kinase kinase activates transcription factors, including Rlm1, that regulate genes for cell wall enzymes. As shown in this study, a two-tiered cascade of functionally redundant protein kinases (Pkh1-Ypk1 and Pkh2-Ypk2) appears to monitor growth of the plasma membrane via a distinct mechanism. In this novel pathway, a product (phytosphingosine) derived from the vesicle-mediated insertion of sphinolipids into the plasma membrane stimulates Pkh1 and Pkh2. These enzymes, in turn, phosphorylate and activate Ypk1 and Ykr2, primarily in the cytosol and the nucleus, respectively. Genetic evidence indicates that Ypk1 and Ykr2 may act through the transcription factor, Smp1, which shares DNA-binding specificity with Rlm1. Nuclear entry is only one potential level at which the activity of these transcription factors might be regulated by these signaling pathways. Cross talk between these pathways probably occurs at two levels: full activation of Pkc1 requires phosphorylation by Pkh1 and/or Pkh2 (Inagaki et al., 1999) and Ypk1 (and perhaps Ykr2) may contribute (directly or indirectly) to Mpk1 activation (Schmelzle et al., 2002). See DISCUSSION for additional details.