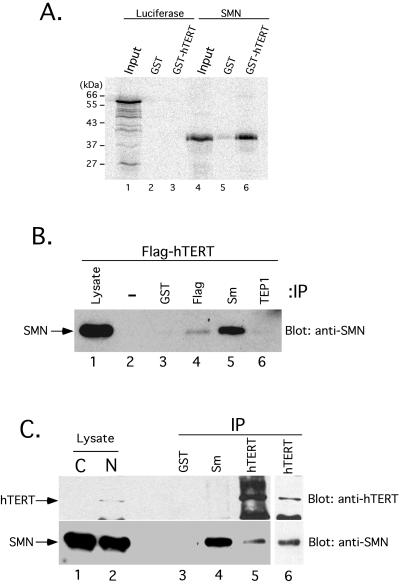
Figure 1.
SMN associates with hTERT in vitro and in vivo. (A) Human telomerase was reconstituted by coexpression of GST-hTERT and hTR in S. cerevisiae as previously described (Bachand and Autexier, 1999). GST (lanes 2 and 5) and GST-hTERT/hTR (lanes 3 and 6) were affinity-purified from equal volumes of yeast extracts and incubated with in vitro–translated [35S]methionine-labeled luciferase (lanes 1–3) and SMN (lanes 4–6). After extensive washing, bound proteins were analyzed by SDS-PAGE and autoradiography. The input lanes (1 and 4) show 5% of the RRL lysate used in the binding reaction. Molecular mass markers are indicated on the left (in kilodaltons, kDa). (B) 293 cells were transiently transfected with a DNA construct expressing FLAG-tagged hTERT. At 20 h after transfection, a total cell lysate was prepared and subjected to immunoprecipitation (IP) without antibody or using anti-GST, anti-FLAG, anti-Sm (Y12), or anti-TEP1. Immunoprecipitates were analyzed by SDS-PAGE and Western blotting for endogenous SMN. The lysate lane corresponds to 5% of the total cell lysate used for the immunoprecipitation. (C) Nucleolar-enriched nuclear extracts were prepared from HeLa cells and subjected to immunoprecipitation (IP) using anti-GST, anti-Sm (Y12), and two different affinity-purified hTERT antibodies. Immunoprecipitates were analyzed by SDS-PAGE and Western blotting for endogenous hTERT (top) and SMN (bottom). Five percent of the cytosolic (C) and nucleolar-enriched nuclear (N) extracts were also loaded.