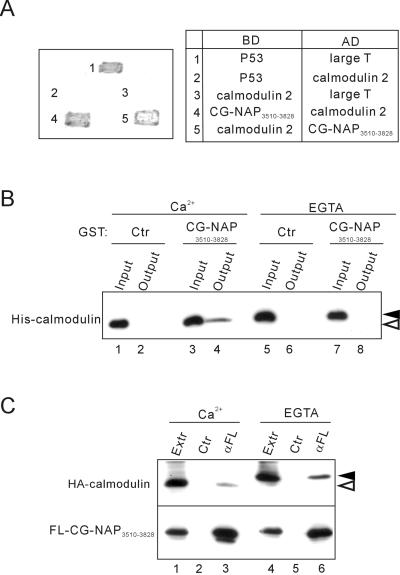
Figure 3.
Association of the centrosomal-localization region CG-NAP3510–3828 with calmodulin. (A) Yeast two-hybrid analysis of interaction between CG-NAP3510–3828 and calmodulin 2. Interaction of the proteins fused to the Gal4 DNA binding domain (BD) and activation domain (AD) was assessed by growth and development of blue color of the transfected yeasts. Combinations of BD and AD constructs are shown on the right. p53 and SV40 large T antigen were used as controls. (B) Direct and Ca2+-dependent binding of CG-NAP3510–3828 with calmodulin. Bacterially expressed His6-tagged calmodulin 2 and GST-tagged CG-NAP3510–3828 were mixed and incubated in the presence of 2 mM CaCl2 or EGTA. After removal of aliquots (Input), glutathione-Sepharose beads were added to the mixture and incubated further, and then the proteins bound to the beads were collected (Output) and immunoblotted with anti-His. Black and white arrowheads indicate the positions of Ca2+-unbound and -bound forms of calmodulin, respectively. (C) Ca2+-independent coimmunoprecipitation of calmodulin with CG-NAP3510–3828. HA-tagged calmodulin 2 and FLAG-tagged CG-NAP3510–3828 were coexpressed in COS7 cells, and then cell extracts (Extr) were prepared in the presence of 2 mM CaCl2 or EGTA. The extracts were immunoprecipitated with anti-FLAG (αFL) or control (Ctr) mouse IgG followed by immunoblot with anti-HA (top) or anti-FLAG (bottom). Black and white arrowheads indicate the positions of calcium-unbound and -bound forms of calmodulin, respectively.