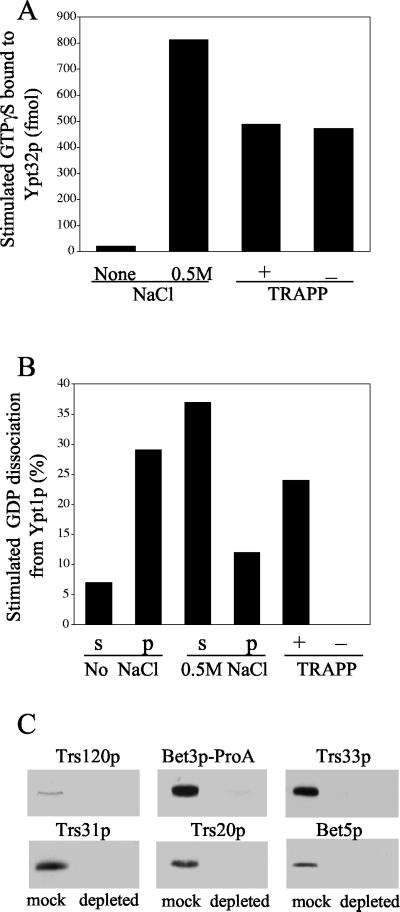
Figure 4.
Depletion of TRAPP does not affect the Ypt32p exchange activity. (A) The P100 fraction of SFNY1088 was treated with buffer (control) or 0.5 M NaCl, centrifuged, and assayed as described below. To deplete TRAPP, the salt-treated supernatant (2.4 mg) was incubated with 20 μl of packed IgG-Sepharose or mock-treated with Sepharose 4B as described in MATERIALS AND METHODS. The TRAPP-depleted or mock-treated sample was incubated with [35S]GTPγS for 30 min at room temperature in the presence or absence of (His6)-Ypt32p. Samples were then incubated with 20 μl of packed Nickel-nitrilotriacetic acid-agarose (Ni-NTA) beads for 1 h at 4°C, and GTPγS uptake was measured. The intrinsic rate of GTPγS uptake onto Ypt32p measured in the presence of BSA, and the value obtained in the absence of (His6)-Ypt32p was subtracted as background. (B) A P100 fraction, prepared from SFNY1088, was treated with buffer (control) or 0.5 M NaCl and centrifuged. The supernatant was incubated with IgG-Sepharose or mock treated with Sepharose 4B. The supernatants (s) and pellets (p) as well as the TRAPP-depleted and mock-treated samples were assayed at 30°C for 30 min for their ability to stimulate the release of [3H]GDP from Ypt1p. The intrinsic rate of [3H]GDP release from Ypt1p was measured in the presence of BSA, and the value obtained was subtracted as background. (C) TRAPP-depleted (0.5 mg) and mock-treated (0.3 mg) samples were resolved by SDS-PAGE and analyzed by Western blot analysis using antibodies directed against Trs120p, Bet3p, Trs33p, Trs31p, Trs20p, and Bet5p.