Abstract
Spirocyclopropanes have become a prevalent structural motif in drug discovery campaigns. However, methods to generate these spirocyclopropanes stereoselectively are scarce, highlighting the need for efficient synthetic methods. In this report, we describe the synthesis of various azaspiro[n.2]alkanes by means of a dirhodium tetracarboxylate catalyzed cyclopropanation of exocyclic olefins using donor/acceptor carbenes. The optimum chiral dirhodium tetracarboxylate catalyst, Rh2(p-PhTPCP)4, results in highly enantioselective cyclopropanation of symmetrical azacyclomethylidenes and highly enantioselective and diastereoselective cyclopropanation of nonsymmetrical azacyclomethylidenes and can achieve up to 83,000 turnovers. Computational studies reveal that the stereoselectivity is controlled by the way the substrate can fit into the chiral pocket generated by the ligands.
Keywords: spiro compounds, asymmetric catalysis, cyclopropanation, catalyst-substrate recognition, dirhodium, carbene


Introduction
The cyclopropyl ring has become a common unit in many pharmaceutical drugs because it has a rigid structure, placing the substituents in a defined position. The cyclopropane motif in many of these drugs are monosubstituted or 1,1-disubstitued as this avoids challenges associated with the synthesis of chiral cyclopropanes. , With greater emphasis by the pharmaceutical industry in recent years to move away from “flat-land” and embrace complex three-dimensional structures, more highly functionalized chiral cyclopropane scaffolds are becoming prevalent in drug candidates. − One particular class of compounds that has generated considerable recent interest has been azaspiro[n.2]alkanes. ,− Representative examples are compounds 1–3 (Figure A). Compound 1, an HDAC inhibitor, showed good potency and high selectivity, particularly after installing the azaspirocyclopropane motif. Similarly, compounds 2 and 3 showed promising activity as a human epidermal growth factor receptor-2 (HER-2) inhibitor and a histamine-3 (H3R) antagonist, respectively. However, the pure enantiomers of 1 and 3 were prepared by chiral resolution, , highlighting the need for enantioselective methods to be developed for this general class of compounds.
1.
Background and current work.
A number of methods have been reported for the synthesis of azaspiro[n.2]alkanes, such as the Corey-Chiakovsky cyclopropanation, phosphorus-mediated ring closure, and metal-catalyzed carbene addition, , but few enantioselective methods are available. One such method was reported by Mendoza in 2019 in which the selective cyclopropanation of exocyclic olefins was obtained using a ruthenium Pheox catalyst (Figure B). Another ruthenium catalyst has been applied to the enantioselective synthesis of a pharmaceutically relevant carbospirocyclopropane, but the reaction required a 5% catalyst loading. The highly selective cyclopropanation with a dirhodium paddle-wheel catalyst to generate a spiro compound was also reported. , However, those studies mainly focused only on symmetrical 1,1-disubstituted alkenes due to diastereoselective challenges of the unsymmetrical one. With the current state of the field, the development of highly diastereoselective and enantioselective methods to readily access highly functionalized azaspiro[n.2]alkanes would greatly enhance the viability of such compounds as potential drug candidates.
Our group has a long-standing interest in rhodium-catalyzed cyclopropanation with donor/acceptor carbenes, and so, we decided to challenge our system to determine if it could be used to achieve stereoselective synthesis of a variety of novel chiral azaspiro[n.2]alkanes. The study explored two classes of compounds: nonsymmetrical azacyclomethylidienes (8, 9) and symmetrical azacyclomethylidenes (7, 10–13) (Figure C). The diastereoselectivity in the first set would be expected to be quite challenging for small transition metal catalysts, because the differentiation between the two substituents on the alkene occurs distal to the alkene. It is likely that secondary interactions away from the reactive site would be required for subtle diastereoselectivity, and this type of feature is more commonly exhibited by enzymes. − We have shown, however, that our bowl-shaped catalysts exhibit subtle selectivity due to interactions between the approaching substrate and the wall of the catalyst, , and thus, there was a reasonable chance our catalysts could achieve good levels of stereoselectivity.
Dirhodium-catalyzed cyclopropanation of monosubstituted alkenes with donor/acceptor carbenes is a well-established process and has even been applied in the scale-up synthesis of drug candidates. − The cyclopropanation of 1,1-diarylethylenes has also been shown to be highly enantioselective, but the extension to other 1,1-disubstituted alkenes has been limited. One of the hallmarks of the cyclopropanation of monosubstituted alkenes is its highly diastereoselective nature. , Furthermore, a series of chiral dirhodium catalysts have been designed so that the reaction can also be achieved with high asymmetric induction. Some of the most notable chiral catalysts are Rh2(DOSP)4, which gives high asymmetric induction when the acceptor group is a methyl ester, Rh2(PTAD)4, which is effective with many types of acceptor groups, Rh2(TCPTAD)4, which is a more rigid version of Rh2(PTAD)4, Rh2(p-PhTPCP)4, which is sterically demanding and capable of operating with low catalyst loading (<0.001 mol %), and the bowl-shaped complex, Rh2(TPPTTL)4, which is capable of inducing unusual selectivity due to interactions between the substrate and the wall of the bowl. , With our broad experience with donor/acceptor carbenes and a series of the most promising chiral catalysts identified, we were well placed to take on the challenge of developing enantioselective approaches to azaspiro[n.2]alkanes.
Results and Discussion
The study began by applying some of our more successful catalysts in a test reaction using the nonsymmetrical N-Boc-3-methylene piperidine (8a) as the substrate with 2,2,2-trichloroethyl 2-(4-bromophenyl)-2-diazoacetate (14a) as the carbene precursor (Table ). The trichloroethyl ester is used because many of our recent catalysts give higher levels of asymmetric induction compared to the corresponding methyl ester. Even though 8a has an allylic methylene site adjacent to the N-Boc group that is activated for C–H functionalization, all of the catalysts were successful at cyclopropanation of 8a because the 1,1-disubstituted alkene is sterically accessible. This is a nice illustration of the influence of steric factors on the cyclopropanation with donor/acceptor carbenes. Rh2(S-DOSP)4 gave low enantioselectivity and diastereoselectivity on the formation of 15a (entry 1). The three C 4-symmetric phthalimido derived catalysts gave higher levels of enantioselectivity (70–86% ee) compared to the reactions with 7, but the diastereoselectivity was still very poor (1.2:1 to 2.4:1 d.r.) (entries 2–4). The only catalyst that gave promising results was Rh2(S-pPhTPCP)4, which generated 15a in 11:1 d.r. with 99% ee.
1. Catalyst Optimization Studies .
Reaction conditions: 14a (0.2 mmol), 8 (1.5 equiv), CH2Cl2, 4 Å molecular sieve. Yields are isolated yields. Enantiomeric excess (ee) was determined by HPLC and SFC analysis.
We assumed that switching the protecting group from the Boc group to the Ts group would enable greater interactions with the catalyst wall because the steric environment around the N-center would increase as the trigonal geometry of carbamate changed to the trigonal pyramidal geometry of sulfonamide. The reactions were repeated using the N-tosyl derivative 8b (entries 6–10). A significant improvement was observed with Rh2(S-pPhTPCP)4, which generated 15b in 80% yield, >20:1 d.r., and 99% ee (entry 10). The relative stereochemistry of the major diastereomer of 15 is as drawn and is readily assigned by means of the distinctive shielding of the methylene group cis to the aryl substituent. On the basis of these optimization studies, it was clear that Rh2(S-pPhTPCP)4 was by far the most superior catalyst for high levels of diastereoselectivity and enantioselectivity.
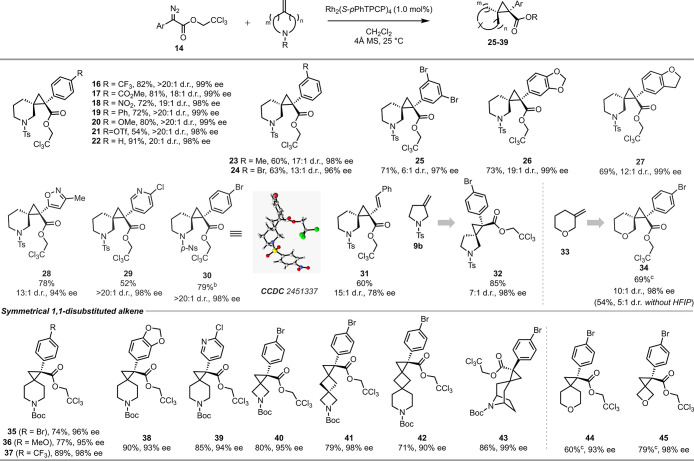
One of the potential advantages of using small transition metal complexes as catalysts over enzymes is that often the transition metal catalysts have a broader substrate scope. Even though the combination of donor/acceptor carbenes and chiral dirhodium catalysts is uniquely suited for highly enantioselective intermolecular reactions, typically a range of functionality can be accommodated on the donor and acceptor groups. In order to determine whether a similar trend is observed here, the Rh2(S-pPhTPCP)4-catalyzed cyclopropanation of 8b was examined with a variety of donor/acceptor carbene precursors, and the results are summarized in Table . The reaction is particularly effective with para-substituted aryldiazoacetates. Both electron-withdrawing and electron-donating groups are accommodated as illustrated in the formation of 17–22 with good diastereocontrol (18 → 20:1 d.r.) and enantiocontrol (98–99% ee). A particularly interesting product is triflate 21 because it is well suited for further diversification. Even an unsubstituted phenyl derivative performed well, generating 22 in 91% yield, 20:1 d.r., and 98% ee. The reaction can also be extended to meta-substituted aryldiazoacetates, albeit with a slight decrease in diastereoselectivity, although it retained high enantioselectivity. This effect is seen with the meta-methyl and meta-bromo derivatives 23 and 24 formed in 17:1 and 13:1 d.r., respectively, but still with high enantioselectivity (96–98% ee). The reaction with a 3,5-dibromo derivative was not as diastereoselective, forming 25 with a 6:1 d.r. The reaction can be extended to heteroaryldiazoacetates, forming 26–29 with good diastereocontrol and enantiocontrol. The reaction to form the pyridyl derivative 29 is particularly impressive, proceeding in >20:1 d.r and 98% ee. The reaction with the aryldiazoacetate 14a was also conducted with the p-nosyl derivative of 8b, and the resulting product 30 was formed in 79% yield, >20:1 d.r., and 98% ee. This reaction was run at a 1.0 mmol scale, and the product readily crystallized. X-ray crystallographic analysis of the product was used to determine the absolute configuration and confirm the assigned relative configuration. The absolute stereochemistry of the other spirocyclopropanes is tentatively assigned by analogy. The vinyl group is another common donor group used in donor/acceptor carbenes. Product 31 was formed with good diastereocontrol (15:1 d.r.), but in this case, there was a considerable drop in the enantioselectivity to 78% ee. The cyclopropanation was also applied to the 5-membered N-tosyl-3-methylenepyrrolidine 9b. The reaction was very effective, generating spirocyclopropane 32 in 85% yield. The enantioselectivity remained very high (98% ee), but the diastereoselectivity (7:1 d.r.) was lower than what had been seen with the six-membered ring homologue 8b. Then, we turned to 3-methylenetetrahydropyran 33, an even more challenging substrate as high diastereoselectivity with 33 requires distinguishing between two sterically similar (CH2 and O) groups. The oxaspiro product 34 is also of significant interest. Initially, the reaction performed moderately to give 34 in 54% yield with a 5:1 d.r. However, upon addition of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as an additive, the reaction performed much better with 69% yield, 10:1 d.r., and 98% ee. We hypothesized that the hydrogen-bonding additive HFIP could interact with the oxygen center of 33, increasing the steric environment around this center and improving the diastereoselectivity.
2. Substrate Scope for Highly Selective Cyclopropanation .
Reaction conditions: 14 (0.2 mmol), alkene (1.5 equiv), CH2Cl2, 4 Å molecular sieve. Yields are isolated yields. Enantiomeric excess (ee) was determined by HPLC and SFC analysis.
The reaction was conducted at 1.0 mmol scale.
1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) (5.0 equiv) was added.
We also examined our optimized conditions with symmetrical exomethylene azacycles because of the interest in this class of compounds. ,,− The reaction with 4-methylenpiperidine 7 generated cyclopropane 35 in 74% yield and 96% ee. Rh2(S-p-PhTPCP)4-catalyzed cyclopropanation is typically tolerant to a range of aryl functionality on the aryldiazoacetates, and this was confirmed to be the case here. The reaction of 7 with aryldiazoacetates 14b–e all proceeded well to form the spirocyclopropanes 36–39 in 77–90% yield with 93–98% ee. Smaller rings such as 3-methylenazetidine 10 were also compatible and generated cyclopropane 40 in 80% yield with 95% ee. The reaction was similarly effective with azaspiro[3.3]heptane 11 and azaspiro[3.5]nonane 12, furnishing the cyclopropanes 41 and 42 in good yield and high asymmetric induction (98 and 90% ee, respectively). The reaction also worked well with the tropane derivative 13, resulting in the formation of 43 in 86% yield and 99% ee, as a signal diastereomer. The reaction can also be applied to 4-methylenetetrahydropyran and 3-methyleneoxetane to provide the oxaspiro cyclopropane products 44 and 45 in good yield (60% and 79%) and enantioselectivity (93% and 98%). HFIP as an additive was again found to be beneficial, presumably because it would hydrogen bond to the oxygen in the starting material and, thus, avoid potential interference.
The cyclopropanations described so far were conducted with 1 mol % of catalyst loading in order to have definitive values for the stereoselectivity by avoiding possible variability in selectivity when using very low catalyst loadings. However, Rh2(S-pPhTPCP)4 has been shown to be effective at very low catalyst loadings in cyclopropanation studies with monosubstituted alkenes. In order to evaluate whether high turnover numbers were equally feasible here, two representative reactions were conducted at low catalyst loadings (Scheme ). The Rh2(S-pPhTPCP)4-catalyzed reaction between 7 and 14a was conducted at 0.001% catalyst loading and generated 33 in 83% yield (83,000 TON) and 90% ee, whereas the reaction with 8b could be achieved with 0.01 mol % to generate 15b in 82% yield (8,200 TON), 14:1 d.r., and 95% ee.
1. Low Catalyst Loading Cyclopropanation .
a Reaction conditions: alkene (1.5 equiv), Rh2(S-pPhTPCP)4, 4 Å molecular sieve at 39 °C.
Computational Study
One of the intriguing features of the current studies is the observation that only Rh2(S-pPhTPCP)4 achieved highly diastereoselective cyclopropanation during the formation of 15b. This is quite different from the cyclopropanation of monosubstituted alkenes with donor/acceptor carbene in which high diastereoselectivity is routine with dirhodium tetracarboxylates catalysts. Therefore, we decided to conduct a computational study to rationalize this finding.
The density functional theory (DFT) calculations were conducted using the [(B3LYP-D3BJ) + CPCM(CH2Cl2)]/[6-31G(d,p) + Lanl2dz (for Rh)] level of theory. As previously reported, Rh2(S-pPhTPCP)4 has a C 2-symmetric structure, in which all four ligands are pointed up with two tilted ligands to create a (α,α′,α,α′) configuration , (Figure A). The carbene preferably binds the top face of the catalyst because of steric reasons to generate two diastereomeric metal-carbene intermediates II and III (Figure B). An interconversion between II and III does not occur under the reaction time scale because the rotational barrier of the ester group is higher than the barrier for cyclopropanation. As the metal-carbene diastereomer II is more stable than III, the further analysis conducted here is focused on the reaction of intermediate II with N-tosyl-3-methylenepiperidine (8b).
2.
Computational studies. (A) X-ray structure of Rh2(S-pPhTPCP)4. (B) DFT-optimized rhodium-carbene intermediate structures. (C) The transition states of cyclopropanation lead to four possible stereoisomers. (D) Distortion-interaction (activation-strain) analysis. (E) Energy decomposition analysis via the sobEDA method provided by the Multiwfn program. (F) Visualization of the noncovalent interaction (NCI) by an independent gradient model based on Hirshfeld partition (IGMH), isovalue = 0.007. The structures were prepared using the VMD program.
First, the enantioselectivity of the reaction was studied by analyzing cyclopropanation transition states TS1 and TS2, resulting in the formation of the major diastereomer (Figure C). Transition state TS2 leading to (S,S)-15b is more energetically favored than TS1 leading to (R,R)-15b by 6.7 kcal/mol, which is in good agreement with the experimental result of 99% ee. A distortion-interaction analysis , was conducted on TS1 and TS2. This analysis decomposed the activation energy into the distortion energy of substrates and metal-carbene fragments (metal-carbene and substrate ) and the interaction energy between them. As we are interested in the differences between TS1 and TS2, we report all energies relative to those for TS2 (Figure D). , The calculations reveal that distortion, particularly metal-carbene distortion, is the most contributing factor in the obtained differences between TS1 and TS2. The identified significant distortion could be attributed to an increasing steric repulsion between the N-tosyl group of 8b and the carbene’s aryl group with the catalyst wall in TS1 (see the RDG map in Figure S3 for more details).
The cyclopropanation transition states TS3 and TS4 resulting in minor diastereomers (R,S)-15b-dr and (S,R)-15b-dr, respectively, were also located. These transition states are higher in energy than TS2 by 5.0 and 3.1 kcal/mol. The distortion-interaction model reveals the distortion of the rhodium-carbene fragment as the most dominant factor for the preference of TS2 over TS3, but the interaction between the rhodium-carbene and substrate fragments is the major factor stabilizing TS2 over TS4. The high distortion in TS3 is again attributed to the increasing steric repulsion between the N-tosyl group of the substrate 8b with the catalyst wall (see Figure S3 for more details). To gain a deeper understanding of the interaction components in TS2 and TS4, the energy decomposition based on dispersion-corrected density functional theory (DFT) (sobEDA) , was used.
The sobEDA analysis decomposed into electrostatic interaction (ΔE els ), Pauli-exchange repulsion (ΔE xrep ), orbital interaction (ΔE orb ), and dispersion energy (ΔE dsip ). The results are shown in Figure E. As seen in this figure, ΔE xrep is significantly higher in TS4 than TS2, which fully reflects the increasing steric repulsion of the carbene’s aryl with the catalyst wall in TS4 (see Figure S3). The dispersion interaction (i.e., ΔE disp ) stabilizes TS2 compared to TS4. The noncovalent interactions contributing to the dispersion energy in TS2 and TS4 were also visualized by an independent gradient model based on Hirshfeld partition (IGMH) (see Figure F). The green surface represents the noncovalent interaction between the substrate and catalyst pocket, while the brighter the color of the atoms, the larger is their contribution to the noncovalent interaction. The IGMH map vividly shows there is much more dispersive interaction in TS2 than in TS4. These computational results cleanly demonstrate that the distal control of Rh2(S-pPhTPCP)4 in cyclopropanation with 8b was achieved by catalyst wall–substrate interaction via a combination of steric repulsion and favorable dispersive interactions as the substrate enters the catalyst pocket.
Conclusion
In conclusion, these studies show that Rh2(S-pPhTPCP)4 is an exceptional chiral catalyst for the enantioselective cyclopropanation of 7–13 with donor/acceptor carbenes. , Not only does the catalyst routinely give high asymmetric induction with a range of substrates, but also it is uniquely suited for high diastereocontrol with substrates that generate two stereogenic centers. Computational studies reveal that the cause of the diastereoselectivity is due to secondary interactions occurring between the catalyst wall and the approaching substrate. The influence of secondary interactions on site selectivity was previously seen in other C4 symmetric bowl-shaped catalysts, , such as Rh2(TPPTTL)4, and the recognition it can also apply to Rh2(S-pPhTPCP)4 offers interesting opportunities for further application in catalyst-controlled reactions. ,
Supplementary Material
Acknowledgments
The experimental work was supported by the National Science Foundation (CHE-1956154) and the National Institute of Health (GM099142 and GM158221). Instrumentation used in this work was supported by the National Science Foundation (CHE 1531620 and CHE 1626172). The computational work was supported by the National Science Foundation under the CCI Centre for Selective C–H Functionalization (CHE-1700982). Constructive discussions within the Catalysis Innovation Consortium facilitated this study. At Emory University, we thank Dr. Bing Wang for NMR measurements, Dr. Fred Strobel for MS measurements, and Dr. John Bacsa for X-ray measurements.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.5c04199.
Deposition Number 2451337 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via the joint Cambridge Crystallographic Data Centre (CCDC) and Fachinformationszentrum Karlsruhe Access Structures service.
‡.
J.K.S. and D.L. contributed equally to this work.
The authors declare the following competing financial interest(s): H.M.L.D. is a named inventor on a patent entitled, Dirhodium Catalyst Compositions and Synthetic Processes Related Thereto (US 8,974,428, issued March 10, 2015).
References
- Menear K. A., Adcock C., Boulter R., Cockcroft X.-l., Copsey L., Cranston A., Dillon K. J., Drzewiecki J., Garman S., Gomez S.. et al. 4-[3-(4-Cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: A Novel Bioavailable Inhibitor of Poly(ADP-ribose) Polymerase-1. J. Med. Chem. 2008;51:6581–6591. doi: 10.1021/jm8001263. [DOI] [PubMed] [Google Scholar]
- Owen D. R., Allerton C. M. N., Anderson A. S., Aschenbrenner L., Avery M., Berritt S., Boras B., Cardin R. D., Carlo A., Coffman K. J.. et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- Lovering F., Bikker J., Humblet C.. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Tice C. M., Singh S. B.. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014;24:3673–3682. doi: 10.1016/j.bmcl.2014.06.081. [DOI] [PubMed] [Google Scholar]
- Talele T. T.. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem. 2016;59:8712–8756. doi: 10.1021/acs.jmedchem.6b00472. [DOI] [PubMed] [Google Scholar]
- Noole A., Sucman N. S., Kabeshov M. A., Kanger T., Macaev F. Z., Malkov A. V.. Highly Enantio- and Diastereoselective Generation of Two Quaternary Centers in Spirocyclopropanation of Oxindole Derivatives. Chem.–Eur. J. 2012;18:14929–14933. doi: 10.1002/chem.201203099. [DOI] [PubMed] [Google Scholar]
- Carreira E. M., Fessard T. C.. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014;114:8257–8322. doi: 10.1021/cr500127b. [DOI] [PubMed] [Google Scholar]
- Ding A., Meazza M., Guo H., Yang J. W., Rios R.. New development in the enantioselective synthesis of spiro compounds. Chem. Soc. Rev. 2018;47:5946–5996. doi: 10.1039/C6CS00825A. [DOI] [PubMed] [Google Scholar]
- Noji S., Hara Y., Miura T., Yamanaka H., Maeda K., Hori A., Yamamoto H., Obika S., Inoue M., Hase Y.. et al. Discovery of a Janus Kinase Inhibitor Bearing a Highly Three-Dimensional Spiro Scaffold: JTE-052 (Delgocitinib) as a New Dermatological Agent to Treat Inflammatory Skin Disorders. J. Med. Chem. 2020;63:7163–7185. doi: 10.1021/acs.jmedchem.0c00450. [DOI] [PubMed] [Google Scholar]
- Hiesinger K., Dar’in D., Proschak E., Krasavin M.. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021;64:150–183. doi: 10.1021/acs.jmedchem.0c01473. [DOI] [PubMed] [Google Scholar]
- Lukin O., Shivanyuk A., Dolgonos G., Gerasov A., Mandzhulo A., Fetyukhin V.. Spirocyclizations of Nortropanes. Synthesis. 2025;57:1375–1401. doi: 10.1055/a-2503-2459. [DOI] [Google Scholar]
- Griffith D. A., Edmonds D. J., Fortin J.-P., Kalgutkar A. S., Kuzmiski J. B., Loria P. M., Saxena A. R., Bagley S. W., Buckeridge C., Curto J. M.. et al. A Small-Molecule Oral Agonist of the Human Glucagon-like Peptide-1 Receptor. J. Med. Chem. 2022;65:8208–8226. doi: 10.1021/acs.jmedchem.1c01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Liu J., Clausen D., Yu Y., Duffy J. L., Wang M., Xu S., Deng L., Suzuki T., Chung C. C.. Discovery of ethyl ketone-based highly selective HDACs 1, 2, 3 inhibitors for HIV latency reactivation with minimum cellular potency serum shift and reduced hERG activity. J. Med. Chem. 2021;64:4709–4729. doi: 10.1021/acs.jmedchem.0c02150. [DOI] [PubMed] [Google Scholar]
- Yao W., Zhuo J., Burns D. M., Xu M., Zhang C., Li Y.-L., Qian D.-Q., He C., Weng L., Shi E.. Discovery of a potent, selective, and orally active human epidermal growth factor receptor-2 sheddase inhibitor for the treatment of cancer. J. Med. Chem. 2007;50:603–606. doi: 10.1021/jm061344o. [DOI] [PubMed] [Google Scholar]
- Brown D. G., Bernstein P. R., Griffin A., Wesolowski S., Labrecque D., Tremblay M. C., Sylvester M., Mauger R., Edwards P. D., Throner S. R.. Discovery of spirofused piperazine and diazepane amides as selective histamine-3 antagonists with in vivo efficacy in a mouse model of cognition. J. Med. Chem. 2014;57:733–758. doi: 10.1021/jm4014828. [DOI] [PubMed] [Google Scholar]
- Malashchuk A., Chernykh A. V., Perebyinis M. Y., Komarov I. V., Grygorenko O. O.. Monoprotected Diamines Derived from 1, 5-Disubstituted (Aza) spiro [2.3] hexane Scaffolds. Eur. J. Org. Chem. 2021;2021:6570–6579. doi: 10.1002/ejoc.202001614. [DOI] [Google Scholar]
- Luo S.-Q., Liu W., Ruan B.-F., Fan S.-L., Zhu H.-X., Tao W., Xiao H.. P (NMe 2) 3 mediated cyclopropanation of α-methylene-β-lactams for rapid syntheses of spirocyclopropyl β-lactams. Org. Biomol. Chem. 2020;18:4599–4603. doi: 10.1039/D0OB00826E. [DOI] [PubMed] [Google Scholar]
- Werth J., Berger K., Uyeda C.. Cobalt catalyzed reductive spirocyclopropanation reactions. Adv. Synth. Catal. 2020;362:348–352. doi: 10.1002/adsc.201901293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul, S. B. D. ; Mookhtiar, K. ; Bhosale, S. ; Kurhade, S. ; Naik, K. ; Velayutham, R. S. V. Spirocyclic Compounds, Compositions and Medicinal Applications Thereof. WO2014054053A1, 2014.
- Wang H., Li Y., Huang L., Xu H., Jiao Y., Zhou Z., Tang Z., Fang F., Zhang X., Ding K.. The synthesis of spirocyclopropane skeletons enabled by Rh (III)-catalyzed enantioselective C–H activation/[4+ 2] annulation. Chem. Catal. 2023;3:100822. doi: 10.1016/j.checat.2023.100822. [DOI] [Google Scholar]
- Montesinos-Magraner M., Costantini M., Ramírez-Contreras R., Muratore M. E., Johansson M. J., Mendoza A.. General Cyclopropane Assembly by Enantioselective Transfer of a Redox-Active Carbene to Aliphatic Olefins. Angew. Chem., Int. Ed. 2019;58:5930–5935. doi: 10.1002/anie.201814123. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu J., Dransfield P. J., Zhu L., Wang Z., Du X., Jiao X., Su Y., Li A.-r., Brown S. P.. Discovery and optimization of potent GPR40 full agonists containing tricyclic spirocycles. ACS Med. Chem. Lett. 2013;4:551–555. doi: 10.1021/ml300427u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha S., Buchsteiner M., Bistoni G., Goddard R., Fürstner A.. A new ligand design based on london dispersion empowers chiral bismuth–rhodium paddlewheel catalysts. J. Am. Chem. Soc. 2021;143:5666–5673. doi: 10.1021/jacs.1c01972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caló F. P., Zimmer A., Bistoni G., Fürstner A.. From serendipity to rational design: Heteroleptic dirhodium amidate complexes for diastereodivergent asymmetric cyclopropanation. J. Am. Chem. Soc. 2022;144:7465–7478. doi: 10.1021/jacs.2c02258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. M., Bruzinski P. R., Lake D. H., Kong N., Fall M. J.. Asymmetric cyclopropanations by rhodium (II) N-(arylsulfonyl) prolinate catalyzed decomposition of vinyldiazomethanes in the presence of alkenes. Practical enantioselective synthesis of the four stereoisomers of 2-phenylcyclopropan-1-amino acid. J. Am. Chem. Soc. 1996;118:6897–6907. doi: 10.1021/ja9604931. [DOI] [Google Scholar]
- Li F., Zhang X., Renata H.. Enzymatic CH functionalizations for natural product synthesis. Curr. Opin. Chem. Biol. 2019;49:25–32. doi: 10.1016/j.cbpa.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. K., Chen K., Huang X., Wohlschlager L., Renata H., Arnold F. H.. Enzymatic assembly of carbon–carbon bonds via iron-catalysed sp 3 C–H functionalization. Nature. 2019;565:67–72. doi: 10.1038/s41586-018-0808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Arnold F. H.. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 2021;54:1209–1225. doi: 10.1021/acs.accounts.0c00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. M., Nagashima T., Klino J. L.. Stereoselectivity of methyl aryldiazoacetate cyclopropanations of 1, 1-diarylethylene. Asymmetric synthesis of a cyclopropyl analogue of tamoxifen. Org. Lett. 2000;2:823–826. doi: 10.1021/ol005563u. [DOI] [PubMed] [Google Scholar]
- Ly D., Bacsa J., Davies H. M.. Rhodium (II)-catalyzed asymmetric cyclopropanation and desymmetrization of [2.2] paracyclophanes. ACS Catal. 2024;14:6423–6431. doi: 10.1021/acscatal.4c01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien J., Davulcu A., DelMonte A. J., Fraunhoffer K. J., Gao Z., Hang C., Hsiao Y., Hu W., Katipally K., Littke A.. The first kilogram synthesis of beclabuvir, an HCV NS5B polymerase inhibitor. Org. Process Res. Dev. 2018;22:1393–1408. doi: 10.1021/acs.oprd.8b00214. [DOI] [Google Scholar]
- Wei B., Sharland J. C., Lin P., Wilkerson-Hill S. M., Fullilove F. A., McKinnon S., Blackmond D. G., Davies H. M.. In situ kinetic studies of Rh (II)-catalyzed asymmetric cyclopropanation with low catalyst loadings. ACS Catal. 2020;10:1161–1170. doi: 10.1021/acscatal.9b04595. [DOI] [Google Scholar]
- Lathrop S. P., Mlinar L. B., Manjrekar O. N., Zhou Y., Harper K. C., Sacia E. R., Higgins M., Bogdan A. R., Wang Z., Richter S. M.. Continuous process to safely manufacture an aryldiazoacetate and its direct use in a dirhodium-catalyzed enantioselective cyclopropanation. Org. Process Res. Dev. 2023;27:90–104. doi: 10.1021/acs.oprd.2c00288. [DOI] [Google Scholar]
- Sharland J. C., Wei B., Hardee D. J., Hodges T. R., Gong W., Voight E. A., Davies H. M.. Asymmetric synthesis of pharmaceutically relevant 1-aryl-2-heteroaryl-and 1, 2-diheteroarylcyclopropane-1-carboxylates. Chem. Sci. 2021;12:11181–11190. doi: 10.1039/D1SC02474D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. M., Liao K.. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 2019;3:347–360. doi: 10.1038/s41570-019-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. M., Hansen T.. Asymmetric intermolecular carbenoid C–H insertions catalyzed by rhodium (ii)(S)-N-(p-dodecylphenyl) sulfonylprolinate. J. Am. Chem. Soc. 1997;119:9075–9076. doi: 10.1021/ja971915p. [DOI] [Google Scholar]
- Reddy R. P., Lee G. H., Davies H. M.. Dirhodium tetracarboxylate derived from adamantylglycine as a chiral catalyst for carbenoid reactions. Org. Lett. 2006;8:3437–3440. doi: 10.1021/ol060893l. [DOI] [PubMed] [Google Scholar]
- Liao K., Pickel T. C., Boyarskikh V., Bacsa J., Musaev D. G., Davies H. M.. Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds. Nature. 2017;551:609–613. doi: 10.1038/nature24641. [DOI] [PubMed] [Google Scholar]
- Fu J., Ren Z., Bacsa J., Musaev D. G., Davies H. M.. Desymmetrization of cyclohexanes by site-and stereoselective C–H functionalization. Nature. 2018;564:395–399. doi: 10.1038/s41586-018-0799-2. [DOI] [PubMed] [Google Scholar]
- Davies H. M.. Finding opportunities from surprises and failures. Development of rhodium-stabilized donor/acceptor carbenes and their application to catalyst-controlled C–H functionalization. J. Org. Chem. 2019;84:12722–12745. doi: 10.1021/acs.joc.9b02428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D., Snodgrass H. M., Gomez C. A., Lewis J. C.. Non-native site-selective enzyme catalysis. Chem. Rev. 2023;123:10381–10431. doi: 10.1021/acs.chemrev.3c00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharland J. C., Dunstan D., Majumdar D., Gao J., Tan K., Malik H. A., Davies H. M. L.. Hexafluoroisopropanol for the Selective Deactivation of Poisonous Nucleophiles Enabling Catalytic Asymmetric Cyclopropanation of Complex Molecules. ACS Catal. 2022;12:12530–12542. doi: 10.1021/acscatal.2c03909. [DOI] [Google Scholar]
- Ren Z., Musaev D. G., Davies H. M.. Key selectivity controlling elements in rhodium-catalyzed C–H functionalization with donor/acceptor carbenes. ACS Catal. 2022;12:13446–13456. doi: 10.1021/acscatal.2c04490. [DOI] [Google Scholar]
- Ren Z., Musaev D. G., Davies H. M.. Influence of Aryl Substituents on the Alignment of Ligands in the Dirhodium Tetrakis (1, 2, 2-Triarylcyclopropane-carboxylate) Catalysts. ChemCatChem. 2021;13:174–179. doi: 10.1002/cctc.202001206. [DOI] [Google Scholar]
- Bickelhaupt F. M., Houk K. N.. Analyzing reaction rates with the distortion/interaction-activation strain model. Angew. Chem., Int. Ed. 2017;56:10070–10086. doi: 10.1002/anie.201701486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Hare S. R., Chen S.-S., Saunders C. M., Tantillo D. J.. C–H Insertion in dirhodium tetracarboxylate-catalyzed reactions despite dynamical tendencies toward fragmentation: implications for reaction efficiency and catalyst design. J. Am. Chem. Soc. 2022;144:17219–17231. doi: 10.1021/jacs.2c07681. [DOI] [PubMed] [Google Scholar]
- Johnson E. R., Keinan S., Mori-Sánchez P., Contreras-García J., Cohen A. J., Yang W.. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010;132:6498–6506. doi: 10.1021/ja100936w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Chen F.. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Lu T., Chen Q.. Simple, efficient, and universal energy decomposition analysis method based on dispersion-corrected density functional theory. J. Phys. Chem. A. 2023;127:7023–7035. doi: 10.1021/acs.jpca.3c04374. [DOI] [PubMed] [Google Scholar]
- Lu T., Chen Q.. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022;43:539–555. doi: 10.1002/jcc.26812. [DOI] [PubMed] [Google Scholar]
- Humphrey W., Dalke A., Schulten K.. VMD: visual molecular dynamics. J. Mol. Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Ly D., Boni Y. T., Korvorapun K., Derdau V., Bacsa J., Musaev D. G., Davies H. M. L.. Impact of Induced Fitting and Secondary Noncovalent Interactions on Site-Selective and Enantioselective C–H Functionalization of Arylcyclohexanes. J. Am. Chem. Soc. 2025;147:23891–23899. doi: 10.1021/jacs.5c06398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During the writing of this manuscript, we became aware of a related study by the Arnold group using evolved enzymes to carry out cyclo-propanations on similar substrates. See: Kennemur, J. L. ; Long, Y. ; Ko, C. J. ; Das, A. ; Arnold, F. H. . Enzymatic Stereodivergent Synthesis of Azaspiro[2.y]alkanes. ChemRxiv 2025; 10.26434/chemrxiv-2025-m7201. (This content is a preprint and has not been peer-reviewed). Both their enzymatic systems and our dirhodium catalysts are capable of highly enantioselective and diastereoselective cyclopropanations using low catalyst loadings, enabling much greater access to enantioenriched spiroazabicyclo[2,n]alkanes. The studies are nicely complementary because the Arnold system works well with an acceptor carbene derived from ethyl diazoacetate, whereas our system is only effective with donor/acceptor carbenes. [DOI]
- A working draft of our study has been submitted to Chem Archive. See Sailer, J. ; Ly, D. ; Wang, A. ; Musaev, D. ; Davies, H. . Enantioselective and Diastereoselective Synthesis of Spiroazabicyclo[2,n]alkanes by Rhodium-Catalyzed Cyclopropanations. ChemRxiv 2025; 10.26434/chemrxiv-2025-5j4tn. This content is a preprint and has not been peer-reviewed. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.