Abstract
This study aimed to probe if a native probiotic Enterococcus faecalis TMBC 10513 could protect the growth and intestinal health of yellow-feathered broilers against avian pathogenic Escherichia coli (APEC) challenge. In vitro bacteriostasis of E. faecalis TMBC 10513 against APEC O1 was initially investigated. Subsequently, 240 one-day-old yellow-feathered female broilers were divided into three groups (eight replicates/group): control group, APEC group (received APEC O1 challenge at 7, 8 and 9 d of age) and EF group (APEC-challenged broilers supplemented with 5 × 10⁸ CFU/kg of E. faecalis TMBC 10513). Parameters were measured at one day post the last gavage (namely d 10). The results revealed that E. faecalis inhibited (P < 0.05) APEC O1 growth and its adhesion to enterocytes. E. faecalis addition alleviated APEC-induced decreases (P < 0.05) in average daily weight gain, average daily feed intake and serum immunoglobulins levels, accompanied by an increase (P < 0.05) in spleen index of broilers. It also attenuated APEC-induced elevations (P < 0.05) in serum d-lactic acid and diamine oxidase levels, together with reductions (P < 0.05) in ileal villus height of broilers. Moreover, E. faecalis addition elevated (P < 0.05) ileal tight junction proteins (ZO-1, Occludin and Claudin-1) and certain cytokines (IL-1β and IL-8) expression, as well as reduced (P < 0.05) ileal APEC O1 amount in APEC-challenged broilers. Remarkably, E. faecalis addition enriched several beneficial bacteria (e.g. Bacteroidota, Actinobacteriota and multiple probiotic candidates) and reduced certain harmful bacteria (e.g. Proteobacteria and Escherichia-Shigella) in the cecum. It also lowered (P < 0.05) the expression of multiple virulence genes of cecal Escherichia. In conclusion, native E. faecalis alleviated APEC-induced intestinal disruptions of broilers at the early stage of infection by directly inhibiting this pathogen as well as improving intestinal immune responses and microbial community, thereby contributing to mitigate the observed growth impairment in APEC-challenged broilers. These findings supply a novel perception into the application of E. faecalis in restricting APEC infection in chicken production.
Keywords: Avian pathogenic Escherichia coli, Broiler, Enterococcus faecalis, Gut microbiota, Intestinal health
Introduction
One of the most common bacterial-related diseases in poultry production is the colibacillosis, as characterized by a series of local or systemic clinical manifestations such as hemorrhage, enteritis and septicemia (Kathayat et al., 2021). These disorders lead to poor growth performance and mortality in poultry (Kathayat et al., 2021; Ren et al., 2024), thus lowering economic benefits of poultry production. As the major inducer of colibacillosis in chickens, avian pathogenic Escherichia coli (APEC) comprises multi-genotypic strains, among which the O1 strain represents one of the most prevalent strains in chicken production (Kathayat et al., 2021). Although APEC can be transmitted via both digestive and respiratory tracts, they mainly colonize the intestine especially the hindgut (Kathayat et al., 2021), probably causing diseases or aggcravating pathological processes in the intestine of chickens particularly those at a young age (Alkie et al., 2019; Schokker et al., 2009). In the past few decades, antibiotics and some other antibacterial drugs had been frequently used to control bacterial infections in animals, however, their usage in feeds has now been banned or restricted globally, due to the developments of bacterial resistance and drug residues. Hence, there is an urgent demand for developing safer and more environmentally-friendly strategies to combat APEC infection in poultry production.
There is increasing interest in regarding probiotics as the potential antibiotic alternatives to limit bacterial infections in poultry (Swelum et al., 2021). As a classic source of probiotic strains, Enterococcus faecalis (E. faecalis) can generate organic acids (such as lactic and acetic acids) to lower microenvironmental pH value, secrete antibacterial substances (e.g. bacteriocin) to destruct pathogenic bacterial cell membrane (Ladjouzi et al., 2025; Yin et al., 2024), as well as competitively inhibit pathogenic colonization of host intestine (Baccouri et al., 2019). The above actions may all contribute to restrain the growth of pathogenic bacteria within the intestine, thus favoring intestinal homeostasis of hosts. Besides, E. faecalis may enhance local and systemic immune responses of hosts probably by stimulating gut-associated lymphoid tissues and/or secreting specific immunoregulatory metabolites (Boeder et al., 2024; Lin et al., 2018). These could also benefit host resistance against bacterial infection. It has been reported that E. faecalis addition could resist pathogenic bacterial colonization as well as improve growth performance and intestinal health in chickens (Hussain et al., 2024; Shehata et al., 2020; Yin et al., 2024). Besides, E. faecalis was found to reinforce intestinal barrier function of chickens against certain pathogen (Clostridium perfringens) invasion (Zhang et al., 2025a). Nevertheless, the putative protecting effects of E. faecalis on growth and intestinal health in chickens against APEC challenge deserve further research. We assumed that probiotic E. faecalis might relieve APEC-induced intestinal disruptions in broilers by eliciting direct bacteriostasis against this pathogen and/or enhancing intestinal immune responses, which are essential for driving hosts to confront bacterial invasion at the early stage of infection (Wang et al., 2023; Withanage et al., 2005). On the other hand, it has been acknowledged that host-derived probiotic strains (namely native probiotics) are commonly superior to non-native probiotic strains (Khongkool et al., 2025). Furthermore, a recent study suggested that the utilization of non-native probiotics even caused potential safety concerns in animals (Zhang et al., 2024). Thereby, the development of native probiotics in chickens is highly warranted. To date, the protective effects of native E. faecalis strains on infected chickens especially those at the early stage of infection are less well studied. Comprehensively, the present study focused on the potential role of native E. faecalis (TMPC 10513) in alleviating APEC-caused growth impairment and intestinal disruptions in yellow-feathered broiler chickens, thus expanding the understanding about the specific application of E. faecalis in limiting bacterial infections in chicken production.
Materials and methods
Bacterial strains
E. faecalis TMPC 10513 preserved in our laboratory was obtained from our previous screening of probiotic strains in the cecum samples of healthy Xin-Xing yellow-feathered broiler chicks. The identified sequences of E. faecalis TMPC 10513 are displayed in Table S1. The APEC O1 strain was obtained from National Center for Veterinary Culture Collection of China.
In Vitro evaluation of the inhibitory effects of E. faecalis TMPC 10513 against APEC O1
Growth Curve. After resuscitation, E. faecalis TMPC 10513 and APEC O1 were inoculated into MRS medium and LB medium, respectively, at a ratio of 1:50 for overnight culture. Thereafter, E. faecalis inoculum was centrifuged at 12,000 g for 3 min, and the resultant supernatant was filtered through a 0.22-μm filter to acquire a sterile extract. Meanwhile, APEC inoculum was centrifuged at 12,000 g for 3 min and washed with sterile phosphate buffer saline (PBS), followed by an adjustment to an OD600 of 1.0 and transferring to LB broth at a ratio of 1:50 for incubation on a shaker. After culture for 2 h, APEC inoculum was added with 1 mL of sterile MRS medium (control group) or added with 0.5 and 1.0 mL of the supernatant extract of E. faecalis inoculum (treatment groups). The OD600 values of APEC inoculum were measured at 2 h intervals until 10 h, based on which the growth curve was plotted and the inhibition of APEC growth by E. faecalis was analyzed.
Inhibition Zone. Following an overnight incubation period, APEC O1 was added to the not yet solidified LB agar at a ratio of 1:1000. Following complete solidification, the plate was punched with Oxford cups and was added with the supernatant extract of E. faecalis. The plate was then placed in an incubator at 37°C for overnight incubation. The size of the inhibition zone was then recorded using a vernier caliper.
Adhesion Ability. Intestinal epithelial cells (IPEC-J2 cells) were seeded in 24-well plate and cultured (37°C, 5 % CO₂) to 90 % confluence. E. faecalis was inoculated in MRS medium to obtain the sterile supernatant extract according to the method described above. APEC was cultured in LB medium until reached the logarithmic phase. The culture was then centrifuged (4000 × g, 10 min) and resuspended in sterile PBS, with the bacterial concentration adjusted to 1 × 10⁹ CFU/mL.
IPEC-J2 cells were washed with sterile PBS and inoculated with 50 μL APEC, followed by addition of 50 μL supernatant extract of E. faecalis or sterile PBS (control). The above mixtures were incubated at 37°C for 1 h, the supernatant was then removed and the cell precipitate was washed with sterile PBS. Afterwards, 1 % Triton X-100 was added to lyse the cell precipitate for 30 min. The resulting lysate was gradually diluted and spread onto MacConkey agar plates, which were then incubated at 37°C for 18 h for colony counting.
Preparation of E. faecalis Additive
faecalis TMPC 10513 was resuscitated, cultured in MRS medium, and adjusted to a concentration of 1 × 10⁹ CFU/mL. The bacterial solution was centrifuged at 12,000 g for 1 min and washed with sterile PBS. Then, the bacterial cells were mixed with 10 % skim milk powder at the volume-to-weight ratio of 1:1 (mL:g) dissoved in sterile PBS and then pre-froze at −80°C for 4 h, followed by freeze-dried in a high-speed freezing centrifuge for 24 h. The resulting freeze-dried powder of E. faecalis TMPC 10513 was enumerated using the plate method, in order to calculate the survival rate of this bacterium after freeze-drying. The survival rate of E. faecalis TMPC 10513 was finally calculated as approximately 90 %, thereby, it could be considered that the amount of viable E. faecalis TMPC 10513 in the prepared additive was 9.0 × 108 CFU/kg.
Design of animal experiments
The animal experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of South China Agricultural University (Guangzhou, China; Approval number: 2024F230). A total of 240 one-day-old female Xin-Xing yellow-feathered broilers were randomly divided into 3 groups: control group (CON), APEC group, and E. faecalis (EF) group. Each group included 8 replicates of 10 broilers in each replicate, with no difference in the initial body weight across all replicates. Broilers in CON and APEC groups were fed the basal diet, while those in EF group received the basal diet supplemented with 0.5 g/kg E. faecalis TMBC 10513 preparation. All broilers were housed in battery cages (10 broilers/cage) in an environmentally controlled room, in which the temperature was kept at approximately 34°C during the initial three days and then reduced by 3°C weekly. Continuous access to the diets and drinking water were ensured throughout the trial. Broilers were exposed to 23 h of light per day. The nutritional composition of the basal diet is exhibited in Table 1. At 7, 8 and 9 d of age, each broiler in APEC and EF groups was gavaged with 2 mL of APEC O1 inoculum (2 × 109 CFU/mL), while each broiler in CON group was gavaged with 2 mL of sterile medium. Note: Intestinal immunological development of broilers generally happens ducring the first two weeks of age (Schokker et al., 2009), and young broilers (no more than two weeks of age) with immature immunological functions in the intestine can be more susceptible to APEC infection (Alkie et al., 2019). Therefore, young broilers (less than 14 d of age) were employed to be challenged by APEC in this study.
Table 1.
Composition of the basal diet (air-dry basis).
| Ingredients | Content (%) |
|---|---|
| Corn | 55.97 |
| Soybean meal | 37.63 |
| Soybean oil | 2.37 |
| Limestone | 1.27 |
| Dicalcium phosphate | 1.75 |
| Sodium chloride | 0.35 |
| L-Lysine. HCl (99 %) | 0.02 |
| DL-Methionine (98 %) | 0.20 |
| Premix1 | 0.24 |
| Total | 100.00 |
| Nutrient levels | |
| Apparent metabolizable energy (MJ/kg) | 12.30 |
| Crude protein | 21.50 |
| Calcium | 0.98 |
| Total phosphorus | 0.66 |
| Toal lysine | 1.26 |
| Total methionine + cysteine | 0.89 |
Premix provided per kilogram of diet: VA 5000 IU, VD3 80.75 mg, VE 31 mg, VK1 1.6 mg, VB1 2.5 mg, VB2 15 mg, VB6 4.0 mg, VB12 0.015 mg, pantothenate 60 mg, nicotinamide 15 mg, biotin 0.05 mg, folic acid 1.5 mg, Choline 450 mg, Cu 9.5 mg, Fe 70 mg, Mn 121 mg, Zn 60 mg, I 1.4 mg, Se 0.45 mg.
Sample collection
At the early stage of infection (1 d post the last gavage, namely 10 d of age), one broiler per each replicate was randomly selected and weighed. Blood sample was collected from the wing vein of each broiler and centrifuged at 3000 × g for 10 min, the serum samples were then collected and preserved at −30°C. Following the collection of blood sample, each broiler was slaughtered to excise the liver, spleen, thymus, bursa of Fabricius and intestine. The midpoint of ileum was harvested and then partitioned into two parts, which were respectively soaked in 4 % paraformaldehyde and froze in liquid nitrogen (followed by storage at −80°C). Finally, cecal content was collected from each broiler.
Determinations of growth performance and organ indexes
At 10 d of age, feed consumption and body weight of broilers were recorded per replicate. The final body weight (FBW), average daily gain (ADG), average daily feed intake (ADFI) together with feed:gain ratio (F/G) during d 1-10 were determined. Besides, the excised organs including the liver, spleen, thymus, and bursa of Fabricius were weighed to determine organ indexes, which were computed as organ weights (g) relative to the live body weight (kg) of each broiler.
Measurement of serum indicators
Serum immunoglobulins (Ig) including IgG, IgA, IgM levels were detected using the enzyme-linked immunosorbent assay kits (Yuanju Bio., Shanghai, China) according to the manufacturer's instructions. Serum d-lactic acid (DLA) level and diamine oxidase (DAO) activity were measured colorimetrically using the commercial assay kits purchased from Abbkine Scientific Co., Ltd (Wuhan, China) and Grace Biotechnology Co., Ltd (Suzhou, China), respectively.
Intestinal morphological examination
Ileum samples fixed in 4 % paraformaldehyde were paraffin-embedded and subjected to hematoxylin-eosin (HE) staining. For each HE-stained section, the representative villi with intact structure were selected for examing intestinal morphological structure using a Motic BA310 microscope equipped with the Image J software. The height of villi (VH), the depth of crypts (CD), and the ratio of VH to CD (VH/CD) of each HE-stained section in each sample were determined according to our previous study (Wang et al., 2023) .
Real-time quantitative PCR (RT-qPCR)
The total RNA was extracted from jejunal and ileal tissues using the FastPure® RNA isolation kits purchased from Vazyme Biotechnology Co., Ltd (Nanjing, China). Meanwhile, cecal bacterial RNA was extracted following the manufacturer’s instructions of a commercial kit purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China). After detection of the concentration and purity using an UV spectrophotometer Nano-Drop 2000 (Thermo Fisher Scientific, Waltham, USA) and validation of the integrity by agarose gel eletrophoresis, the extracted RNA samples were reverse-transcribed into cDNA samples using the reverse transcription kit (EZBioscience Co., Ltd, Suzhou, China). The SYBR qPCR master mix (MIKX Co., Ltd, Shenzhen, China) was used for RT-qPCR that was performed on an Archimed-X4 Real Time PCR instrument (RocGene, Beijing, China). Finally, the relative mRNA expression of the target genes was calculated using the 2-ΔΔCt method, with glyceraldehyde-3-phosphate dehydrogenase serving as the internal reference for broilers and DNA-directed RNA polymerase subunit alpha serving as the internal reference for Escherichia. The primer sequences used in this experiment are shown in Table 2.
Table 2.
Primer sequences for real-time PCR.
| Genes | Primer sequences (5′−3′) | Product size (bp) | |
|---|---|---|---|
| Chicken | GAPDH | F: TCGCATIGTTTTCCGTGCTG | 75 |
| R: TCAGCGTCTAACAGGTGCTT | |||
| ZO-1 | F: GAGTTTGTATGTGGCGTT | 298 | |
| R: GTGGGAGGATGCTGTTGT | |||
| Occludin | F: ATCAACAAAGGCAACTCT | 157 | |
| R: GCAGCAGCCATGTACTCT | |||
| Claudin-1 | F: GTGCAGAAGATGCGGATGG | 253 | |
| R: TTGGTTTGGGAAGATGTTGTTT | |||
| TNF-α | F: GCATCGCCGTCTCCTACCA | 204 | |
| R:CCTGCCCAGATTCAGCAAAGT | |||
| IL-1β | F: TGCCCTGCAGAAGAAGCCTCG | 204 | |
| R: GACGGGCTCAAAAACCTCCT | |||
| IL-8 | F: TGAGAAGCAACAACAACAGCA | 129 | |
| R: CAGCACAGGAATGAGGCATA | |||
| Escherichia | rpoA | F: GCACCAAAGAAGGCGTTCAG | 139 |
| R: ATATCGGCTGCAGTCACAGG | |||
| hycA | F: CGGCCATGATTGATGGCAAGG | 100 | |
| R: GGCGGTGTATAAGCTGTCGT | |||
| csgD | F: ACTGGCCTCATATCAACGGC | 98 | |
| R: CGTAAAGTAGCATTCGCCGC | |||
| luxS | F: TTGGTACGCCAGATGAGCAG | 113 | |
| R: GCCACACTGGTAGACGTTCA | |||
| relA | F: GTTCGCCGGATGTTATTGGC | 100 | |
| R: CCGGCGCATCTTTTACTTCG | |||
| ompR | F: GCGTCGCTAATGCAGAACAG | 142 | |
| R: ATGATCGGCATCGGATTGCT | |||
| APEC O1 | F: CGATTTGAGCGCAAGGTTG | 263 | |
| R: CATTAGGTGTCTGGCCACG |
1GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ZO-1, zonula occluden 1; TNF-α, tumor necrosis factor α; IL, interleukin; rpoA, DNA-directed RNA polymerase subunit alpha; hycA, formate hydrogenlyase regulator HycA; csgD, curli subunit gene D; luxS, S-ribosylhomocysteine lyase; relA, (p)ppGpp synthetase gene; ompR, DNA-binding dual transcriptional regulator OmpR; APEC, avian pathogenic Escherichia coli.
Measurement of intestinal APEC O1 amount
APEC amount in the intestine was determined by the microbial copy number method as described in our previous study (Ren et al., 2024). Briefly, total genomic DNA was extracted from ileal tissue, and the extracted DNA was utilized as a template for PCR amplification using APEC O1-specific primers (Table 2). The amplified products were recovered, diluted at a concentration of 10-fold, and employed as a standard for the absolute quantification of APEC O1 colonization of the ileum. The copy number of target microbe was calculated based on the standard curve.
Gut microbial analysis
The bacterial genomic DNA was extracted from cecal content in accordance with the instructions of a commercial kit (Solarbio Science & Technology Co., Ltd., Beijing, China). Following the detection of concentration and quality as described above, 16S rDNA sequences spanning the V3-V4 variable regions were amplified using the primer pairs 338F (5′-ACTCCTACGGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The resultant PCR products were purified, quantified and homogenized, and then used to construct a sequencing library. The high-throughput sequencing (2 × 250 bp) was performed on an Illumina Novaseq 6000 instrument (Illumina, San Diego, USA). The subsequent bioinformatics analysis was performed referring to our previous research (Wang et al., 2023).
Data Analysis
The significance of the differences between two groups was analyzed using the Student's t-test of SPSS 26.0 software. The data from more than two groups were analyzed using the one-way ANOVA, and the differences among groups were detected using the Tukey's multiple comparisons. The results are presented as mean ± standard error. P < 0.05 was considered to be statistically significant.
Results
APEC proliferation and adhesion capacity
The supernatant extract of E. faecalis inhibited (P < 0.05) the growth of APEC O1 (Fig. 1A) and its adhesion capacity to IPEC-J2 cells (Fig. 1B). In addition, E. faecalis supernatant reached a inhibition zone of 15.1 ± 0.45 mm against APEC O1 (Fig. 1C).
Fig. 1.
Effects of the supernatant extract of Enterococcus faecalis TMBC 10513 inoculum on the growth (A) and adhesion ability (B) of avian pathogenic Escherichia coli (APEC) O1 as well as its inhibition zone against APEC O1 (C). a-c Values with different letters differ significantly (P < 0.05). CON, control (LB medium); 0.5 mL and 1.0 mL indicate the additive amount of the supernatant extract of E. faecalis TMPC 10513 inoculum; EF, supernatant extract of E. faecalis TMPC 10513 inoculum.
Growth performance
FBW, ADG and ADFI were lower (P < 0.05) in APEC group than those in CON group (Table 3). FBW and ADG rather than ADFI were higher (P < 0.05) in EF group than those in APEC group, whilst F/G showed no difference (P > 0.05) among groups.
Table 3.
Effects of Enterococcus faecalis TMBC 10513 addition on growth performance1 of avian pathogenic Escherichia coli (APEC) O1-challenged broilers (d 1-10).
| CON2 | APEC | EF | P-value | |
|---|---|---|---|---|
| IBW (g) | 38.73±0.05 | 38.71±0.03 | 38.71±0.04 | 0.918 |
| FBW (g) | 116.86±1.79a | 104.88±1.1b | 112.85±1.98a | <0.001 |
| ADG (g) | 7.81±0.18a | 6.55±0.11c | 7.1 ± 0.25b | <0.001 |
| ADFI (g) | 11.36±0.14a | 9.92±0.14b | 9.54±0.25b | <0.001 |
| F/G | 1.49±0.02 | 1.49±0.02 | 1.41±0.03 | 0.504 |
Values within a row with different superscript letters differ significantly (P < 0.05).
IBW, initial body weight; FBW, final body weight; ADG, average daily gain; ADFI, average daily feed intake; F/G, feed-to-gain ratio.
CON, control (broilers received a basal diet and were gavaged with sterile medium); APEC, broilers received a basal diet and were gavaged with APEC O1 (4 × 109 CFU/bird) at 7, 8 and 9 d of age; EF, APEC-challenged broilers supplemented with 5 × 108 CFU/kg E. faecalis TMBC 10513.
Organ indexes
As presented in Table 4, spleen index was higher (P < 0.05) in APEC group than that in CON group, but it was lower (P < 0.05) in EF group versus APEC group. Liver index, thymus index and bursa of Fabricius index remained similar (P > 0.05) among groups.
Table 4.
Effects of Enterococcus faecalis TMBC 10513 addition on organ indexes of avian pathogenic Escherichia coli (APEC) O1-challenged broilers (d 10).
| CON1 | APEC | EF | P-value | |
|---|---|---|---|---|
| Liver index (g/kg) | 34.74±1.1 | 38.93±1.26 | 35.36±1.3 | 0.107 |
| Spleen index (g/kg) | 1.31±0.08b | 1.63±0.02a | 1.26±0.06b | 0.020 |
| Thymus index (g/kg) | 3.05±0.08 | 3.28±0.23 | 3.16±0.12 | 0.553 |
| Bursa of Fabricius index (g/kg) | 1.68±0.17 | 1.48±0.11 | 1.79±0.15 | 0.603 |
Values within a row with different superscript letters differ significantly (P < 0.05).
CON, control (broilers received a basal diet and were gavaged with sterile medium); APEC, broilers received a basal diet and were gavaged with APEC O1 (4 × 109 CFU/bird) at 7, 8 and 9 d of age; EF, APEC-challenged broilers supplemented with 5 × 108 CFU/kg E. faecalis TMBC 10513.
Serum indicators
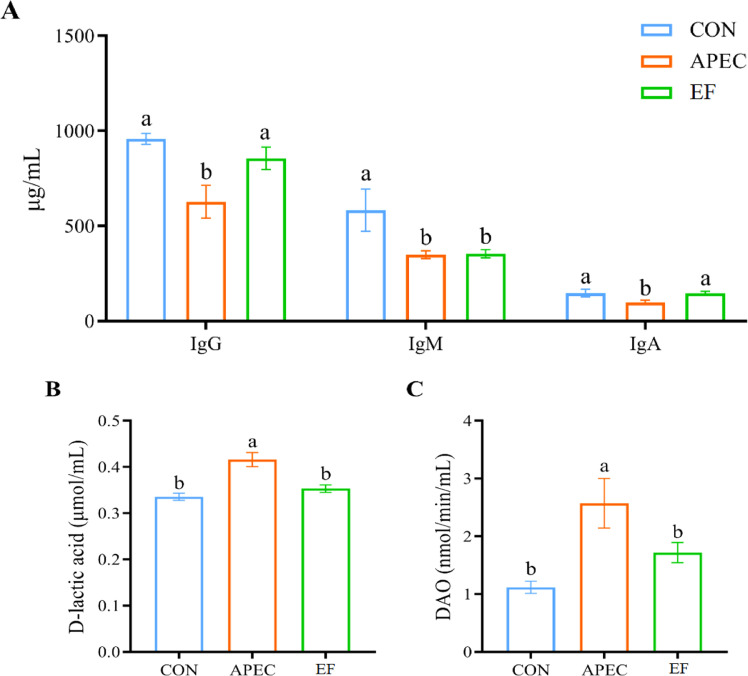
APEC group had lower (P < 0.05) serum IgG, IgM and IgA levels relative to CON group (Fig. 2A). However, the above indicators except serum IgM level in EF group were higher (P < 0.05) than APEC group and similar to (P > 0.05) CON group. Serum DLA and DAO levels were higher (P < 0.05) in APEC group versus CON group (Fig.s 2B, C), but these indicators did not (P > 0.05) differ between EF group and CON group.
Fig. 2.
Effects of Enterococcus faecalis (E. faecalis) TMPC 10513 addition on serum parameters of avian pathogenic Escherichia coli (APEC) O1-challenged broilers (d 10). a,b Values with different letters differ significantly (P < 0.05). CON, control (broilers received a basal diet and were gavaged with sterile medium); APEC, broilers received a basal diet and were gavaged with APEC O1 (4 × 109 CFU/bird) at 7, 8 and 9 d of age; EF, APEC-challenged broilers supplemented with 5 × 108 CFU/kg E. faecalis TMBC 10513.
Intestinal mucosal morphology
There were reductions (P < 0.05) in ileal VH in APEC group when compared with CON group (Table 5), but these parameters were similar (P > 0.05) between EF group and CON group. No differences (P > 0.05) were observed in CD and VH/CD of ileum among groups.
Table 5.
Effects of Enterococcus faecalis TMBC 10513 addition on ileal mucosal morphology1 of avian pathogenic Escherichia coli (APEC) O1-challenged broilers (d 10).
| CON2 | APEC | EF | P-value | |
|---|---|---|---|---|
| VH (μm) | 662.64±25.47a | 549.89±28.65b | 612.80±17.88a,b | 0.029 |
| CD (μm) | 180.89±17.88 | 193.79±14.85 | 162.67±23.97 | 0.514 |
| VH/CD | 3.76±0.35 | 2.91±0.28 | 4.08±0.71 | 0.207 |
Values within a row with different superscript letters differ significantly (P < 0.05).
VH, villus height; CD, crypt depth; VH/CD, villus height to crypt depth ratio.
CON, control (broilers received a basal diet and were gavaged with sterile medium); APEC, broilers received a basal diet and were gavaged with APEC O1 (4 × 109 CFU/bird) at 7, 8 and 9 d of age; EF, APEC-challenged broilers supplemented with 5 × 108 CFU/kg E. faecalis TMBC 10513.
Relative mRNA expression of intestinal genes
Compared with CON group, APEC group showed no changes (P > 0.05) in the expression levels of tight junction (TJ) proteins (ZO-1, Occludin and Claudin-1) and cytokines (IL-1β, IL-8 and TNF-α) in ileum (Fig. 3). However, the above parameters except ileal TNF-α expression were higher (P < 0.05) in EF group than those in APEC group.
Fig. 3.
Effects of Enterococcus faecalis (E. faecalis) TMPC 10513 addition on the relative mRNA expression levels of ileal tight junction proteins (A) and cytokines (B) of avian pathogenic Escherichia coli (APEC) O1-challenged broilers (d 10). a,b Values with different letters differ significantly (P < 0.05). CON, control (broilers received a basal diet and were gavaged with sterile medium); APEC, broilers received a basal diet and were gavaged with APEC O1 (4 × 109 CFU/bird) at 7, 8 and 9 d of age; EF, APEC-challenged broilers supplemented with 5 × 108 CFU/kg E. faecalis TMBC 10513.
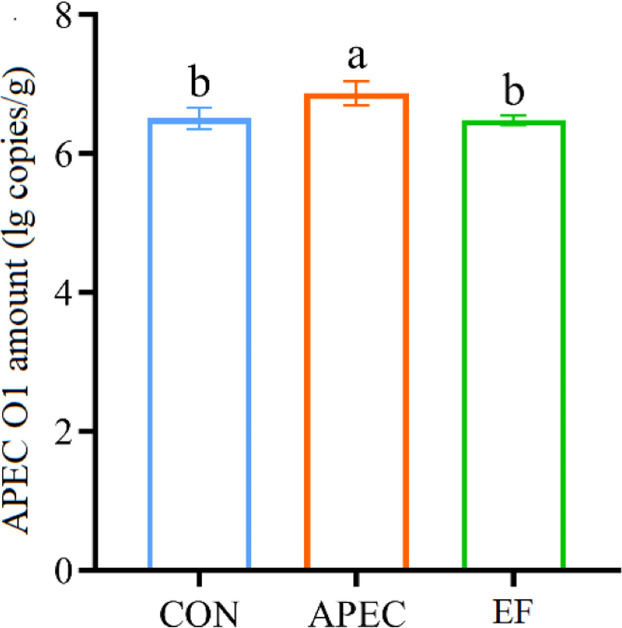
Intestinal APEC O1 amount
APEC group showed an increase (P < 0.05) in ileal APEC O1 amount compared with CON group (Fig. 4). However, ileal APEC O1 amount in EF group was lower (P < 0.05) than APEC group and similar to (P > 0.05) CON group.
Fig. 4.
Effect of Enterococcus faecalis (E. faecalis) TMPC 10513 addition on avian pathogenic Escherichia coli (APEC) O1 amount in the ileum of APEC O1-challenged broilers (d 10). a,b Values with different letters differ significantly (P < 0.05). CON, control (broilers received a basal diet and were gavaged with sterile medium); APEC, broilers received a basal diet and were gavaged with APEC O1 (4 × 109 CFU/bird) at 7, 8 and 9 d of age; EF, APEC-challenged broilers supplemented with 5 × 108 CFU/kg E. faecalis TMBC 10513.
Intestinal microbial structure
The α-diversity parameters (ACE and Chao 1 indexes) of cecal microbiota in EF group were higher than those in other two groups (Fig. S1), although they did not reach the level of significance (P > 0.05). Principal coordinates analysis (PCoA) revealed a separation (P < 0.05) in cecal microbial composition among groups (Fig. 5A). The dominant phyla in the cecum were Firmicutes, Bacteroidota, Proteobacteria, and Actinobacteriota (Fig. 5B). Compared with CON group, APEC group had increased abundances of Firmicutes and Proteobacteria along with reduced abundances of Bacteroidota and Actinobacteriota. However, the above changes of bacterial abundances were alleviated in EF group. At the genus level, Alistipes, Faecalibacterium, Parabacteroides, Bacteroides, and Escherichia-Shigella were the dominators in the cecum. Compared to CON group, APEC group exhibited increases in the abundances of Parabacteroides and Escherichia-Shigella, in contrast to the variation trend of Bacteroides. However, the abundances of Faecalibacterium, Parabacteroides and Escherichia-Shigella were lower while the abundances of Alistipes and Bacteroides were higher in EF group versus APEC group.
Fig. 5.
Effects of Enterococcus faecalis TMPC 10513 addition on the β-diversity (A) and bacterial distribution (B) of cecal microbiota of avian pathogenic Escherichia coli (APEC) O1-challenged broilers (d 10). CON, control (broilers received a basal diet and were gavaged with sterile medium); APEC, broilers received a basal diet and were gavaged with APEC O1 (4 × 109 CFU/bird) at 7, 8 and 9 d of age; EF, APEC-challenged broilers supplemented with 5 × 108 CFU/kg E. faecalis TMBC 10513.
The linear discriminant analysis (LDA) combined with effect size measurements (LEfSe) analysis was further used to detect bacterial enrichments (LDA > 3.0, P < 0.05) among groups (Fig. 6). The results showed that CON and APEC groups were enriched with little identified bacteria. Comparatively, EF group was enriched with Actinobacteriota and several probiotic candidates such as Parasutterella, Ruminococcaceae bacterium, Blautia massiliensis, Lactobacillus kitasatonis, and Agathobaculum desmolans.
Fig. 6.
Effect of Enterococcus faecalis TMPC 10513 addition on microbial enrichment in the cecum of avian pathogenic Escherichia coli (APEC) O1-challenged broilers (d 10). LDA, linear discriminant analysis. CON, control (broilers received a basal diet and were gavaged with sterile medium); APEC, broilers received a basal diet and were gavaged with APEC O1 (4 × 109 CFU/bird) at 7, 8 and 9 d of age; EF, APEC-challenged broilers supplemented with 5 × 108 CFU/kg E. faecalis TMBC 10513.
Relative mRNA expression of cecal escherichia virulence genes
Because of the difference in the abundance of cecal Escherichia-Shigella (the classic harmful bacteria) among groups, we further determined the relative mRNA expression profile of virulence genes of cecal Escherichia (one of the dominated bacterium in chicken gut). As illustrated in Fig. 7, the expression levels of virulence genes including csgD, hycA, relA and omoR were higher (P < 0.05) in APEC group relative to CON group, but these genes expression levels in EF group were lower (P < 0.05) than those in APEC group and not different (P > 0.05) from CON group. Furthermore, EF group had a lower (P < 0.05) expression of luxS than that of APEC group.
Fig. 7.
Effects of Enterococcus faecalis TMPC10513 addition on the relative mRNA expression levels of cecal Escherichia virulence genes of avian pathogenic Escherichia coli (APEC) O1-challenged broilers (d 10). a,b Values with different letters differ significantly (P < 0.05). CON, control (broilers received a basal diet and were gavaged with sterile medium); APEC, broilers received a basal diet and were gavaged with APEC O1 (4 × 109 CFU/bird) at 7, 8 and 9 d of age; EF, APEC-challenged broilers supplemented with 5 × 108 CFU/kg E. faecalis TMBC 10513.
Discussion
As the primary inducer of avian colibacillosis, APEC has been evidenced to impair growth performance in chickens (Ren et al., 2024). Likewise, this study confirmed that APEC-challenged broilers displayed impaired growth performance, as exhibited by the decreases in FBW, ADG and ADFI. Numerous studies have revealed the beneficial effects of E. faecalis on growth performance in broilers (Hussain et al., 2024; Shehata et al., 2020; Zhang et al., 2025a), contrasting to the results described elsewhere (Song et al., 2016; Yin et al., 2024). The discrepancies might be connected with the probiotic strain, broiler breed and health status. In this study, we found that supplementation of E. faecalis alleviated APEC-caused impairments in growth performance by increasing ADG and FBW in broilers, which could be ascribed to the observed roles of this probiotic in protecting the intestine of broilers against APEC challenge (Diaz Carrasco et al., 2019).
Organ index is an important indicator of immune function and health status in broilers (Wang et al., 2023). Among the organs, the spleen is known as an immune organ to mediate systemic immune responses to bacterial infection through various immune cells such as plasmocytes (a producer of immunoglobulins/antibodies). Consistent with previous studies (Song et al., 2023; Wu et al., 2022), we found that APEC challenge elevated spleen index but reduced serum immunoglobulins (IgG, IgM and IgA) levels in broilers. It was probable that the virulence factors such as ectodomain proteins of the invaded APEC impeded cellular differentiation and antibody secretion of host (Yang et al., 2025; Zhu et al., 2024), which suppressed systemic humoral immune responses and subsequently triggered compensatory pathological enlargement of the spleen (Meng et al., 2024; Song et al., 2023). Previously, E. faecalis addition was reported to enhance humoral immunity by increasing serum immunoglobulins levels in broilers under non-challenged condition (Shehata et al., 2020; Yin et al., 2024). In this study, E. faecalis addition counteracted APEC-caused decreases in serum IgG and IgA levels along with elevation in spleen index in broilers. We speculated that E. faecalis fortified systemic immunity in broilers against APEC challenge probably through stimulating gut-associated lymphoid tissues (Wan et al., 2016; Boeder et al., 2024). This action could reduce the demand for antibody (immunoglobulins) production by the plasmocytes in the spleen and subsequently restored the elevation of spleen index in APEC-challenged broilers.
Intestinal mucosal morphology represented by the villus-crypt architecture is the core structural components of the intestine. Ameliorations in intestinal mucosal morphology such as the increases in VH and VH/CD are advantageous for intestinal digestion and absorption as well as epithelial barrier, therefore protecting against bacterial invasion and benefiting growth performance of chickens (Wang et al., 2023). Consistent with previous study (Ren et al., 2024), the present study revealed that APEC challenge caused reduction of ileal VH of broilers, but it was alleviated by E. faecalis addition. This result was analogous to a previous study in piglets (Wang et al., 2019) and revealed a potential of E. faecalis to protect intestinal absorption and barrier in broilers against APEC, which might thus partly account for the observed protective effects of this probiotic on growth performance in APEC-challenged broilers. Intestinal TJ are cross-linking structures between intestinal epithelial cells and consist of certain unique proteins (such as claudins, occludin, and ZO families), which sustain intestinal integrity to creat paracellular permeability barrier of the intestine (Wang et al., 2023). In line with our previous study (Ren et al., 2024), this study showed that APEC challenge did not change the expression of ileal TJ proteins in broilers. However, it increased the levels of serum DAO and DLA, which are the intracellular enzyme and bacterial metabolite, respectively, within the intestine, serving as pivotal indicators of intestinal permeability barrier (Fan et al., 2024; Wang et al., 2023). These results confirmed a destruction of intestinal barrier in broilers induced by APEC challenge (Wu et al., 2022). It was possible that APEC-induced intestinal barrier destruction was originated from other factors, such as disruption of intestinal villi and redistribution of TJ proteins (Hurtado-Monzón et al., 2024). Previous study has indicated a role of E. faecalis in benefiting intestinal barrier in broilers (Zhang et al., 2025a). Analogously, we herein detected that supplementing E. faecalis to APEC-challenged broilers elevated ileal TJ proteins (ZO-1, Occludin, and Claudin-1) expression, which corresponded to the observed ability of it to reduce serum DLA and DAO levels. These findings underscored a role of E. faecalis in reinforcing intestinal barrier of APEC-challenged broilers.
The expression profiles of intestinal cytokines are closely associated with intestinal health, and often exhibit complicated responses to bacterial infection in broilers (Alkie et al., 2019). In general, the increased expression of inflammatory cytokines in the intestine at the early stage of infection is momentous for promoting the elimination of pathogens by intestinal immune cells (Alkie et al., 2019; Wang et al., 2023), in order to avoid the deterioration of cellular invasion. Strikingly, this study showed that APEC challenge did not alter the expression of ileal inflammatory cytokines (IL-1β, IL-8 and TNF-α) in broilers, implying that APEC inactivated intestinal immune responses at the early stage of infection and then compromised intestinal barrier function (Ruchaud-Sparagano et al., 2007). Comparatively, supplementing E. faecalis to APEC-challenged broilers increased ileal IL-1β and IL-8 expression, but without causing damages of ileal structure (such as epithelial morphology and TJ). These results suggested that E. faecalis enhanced intestinal immune reactions (other than led to intestinal inflammatory injuries) in broilers at the early stage of APEC infection, which corresponded to the observed role of this probiotic in elevating serum immunoglobulins levels in APEC-challenged broilers. Similarly, previous studies also discovered that probiotic E. faecalis could prime host immune responses by promoting inflammatory cytokines expression (Agnieszka and Jarzembowski, 2024; Mi et al., 2023). It could be inferred that the enhancement of intestinal immunity by E. faecalis accelerated the clearance of intestinal APEC, thereby conducing to protect broiler intestine against infection (Pan et al., 2023). In support of this inference, we further observed that E. faecalis addition reversed APEC-caused increase in ileal APEC amount of broilers. Of note, the above benefit might not only originate from the enhancement of intestinal immunity, but might also derive from other mechanisms such as the direct bacteriostasis. Indeed, we detected that E. faecalis directly inhibited APEC growth and its adhesion to enterocytesc, verifying a direct bacteriostasis action of this probiotic against APEC. This action was likely responsible by the capacity of this probiotic to secrete bactericidal substances such as organic acids and bacteriocins (Ladjouzi et al., 2025; Yin et al., 2024). Overall, we proposed that E. faecalis addition reduced intestinal APEC amount in broilers by exerting direct bacteriostasis against APEC and fortifying intestinal immunity, thus favoring to alleviate intestinal disruptions caused by APEC.
Gut microbiota can mediate intestinal pathological processes related to bacterial infections (Ferreira et al., 2011), as well as being closely associated with intestinal homeostasis (including absorption, barrier and immune functions) and growth performance in chickens (Diaz Carrasco et al., 2019; Wang et al., 2023). In accordance with our previous study (Ren et al., 2023), this study corroborated that APEC challenge perturbed gut microbial composition of broilers at multiple taxonomic levels. However, these perturbations were mitigated by E. faecalis addition, which was similar to some previous studies where E. faecalis addition improved gut microbial composition in chickens (Hussain et al., 2024; Shehata et al., 2020). Among the dominated phyla changed by E. faecalis, Proteobacteria that contains considerable harmful bacteria plays negative roles while Actinobacteriota (inclusion of plentiful producers of bioactive substances) plays positive roles in maintaining intestinal homeostasis (Binda et al., 2018; Litvak et al., 2017). Besides, the increase in Firmicutes with the simultaneous decrease in Bacteroidota may mediate the occurrence and development of intestinal pathological changes (Qi et al., 2021; Yin et al., 2025). Among the dominated genera changed by E. faecalis, Alistipes can improve intestinal diseases by protecting intestinal epithelia (Lin et al., 2025). Parabacteroides exerts double-edged actions in the intestine (Cui et al., 2022), while Bacteroides possesses polysaccharide utilization loci and represents a critical producer of short-chain fatty acids that can protect gut health through various ways (Cheng et al., 2021). Conversely, Escherichia-Shigella represent the typical harmful bacteria with a capacity to disrupt intestinal structure (dos Reis and Horn, 2010). In this study, supplementing E. faecalis to APEC-challenged broilers reduced the abundances of dominated phyla (Firmicutes and Proteobacteria) and genus (Escherichia-Shigella), as well as elevated the abundances of other dominated phyla (Bacteroidota and Actinobacteriota) and genera (Alistipes and Bacteroides), which could conduce to attenuate intestinal disruptions in APEC-challenged broilers. LEfSe analysis further revealed that E. faecalis addition enriched several probiotic candidates such as Parasutterella, Ruminococcaceae bacterium, Blautia massiliensis, Lactobacillus kitasatonis and Agathobaculum desmolans in the cecum, which might be due to the cross-feeding mechanism in gut microbes (Culp and Goodman, 2023). Among the above microbes, Parasutterella and Ruminococcaceae can benefit gut health by improving intestinal barrier and metabolic dysfunction (An et al., 2025). Blautia massiliensis may promote health recovery by regulating host metabolism and immune responses (Li et al., 2025). Lactobacillus kitasatonis has been identified as a probiotic candidate in chicken gut with excellent stress-resistance and strong capacity to antagonize certain pathogen (Zhang et al., 2025b), Agathobaculum desmolans has been recognized as a potential producer of butyrate (Ahn et al., 2016), which is well-known as a key nutrient for enterocytes, impelling the renewal and repairing of intestinal epithelia as well as boosting intestinal mucosal immunity against pathogens (Bedford and Gong, 2018). Accordingly, the enrichments of the above probiotic candidates in gut could also be conducive to the observed role of E. faecalis in mitigating intestinal disruptions in APEC-challenged broilers.
One of the mechanims for gut microbiota interacting with host is the expression of virulence factors (Kathayat et al., 2021), which have been evidenced to benefit bacterial pathogenicity and exacerbate intestinal damages in broilers (Ren et al., 2024). Because E. faecalis was found to attenuate the increased abundance of classic harmful bacteria Escherichia-Shigella in the cecum of APEC-challenged broilers, we then determined if this probiotic could further repress the expression of virulence factors-encoding genes of cecal Escherichia (one of the dominated bacterium in chicken gut). As expected, supplementing E. faecalis to APEC-challenged broilers moderated the increased expression of the virulence genes csgD, hycA, relA and ompR, as well as reduced the luxS expression. Among these genes, the csgD encodes an essential subunit of curli pilus that propels the motility, adherence and biofilm formation of Escherichia, thus playing a critical role in initiating infection for broilers (Kathayat et al., 2021). The hycA encodes an antibiotic resistance-associated protein in Escherichia (Aunins et al., 2020). The relA and luxS encode a ribosome-related enzyme (diphosphokinase of guanosine triphosphate) and a synthetase of autoinducer-2 (a key quorum-sensing signal molecule), respectively, of bacteria; while the ompR codes for a response modulator of bacterial two-component system EnvZ/OmpR (denoted as a signal transducer). The above-mentioned virulence genes are all beneficial for strengthening the potential pathogenicitcy (including the survival, adaptability, adhesion and invasion) of Escherichia (Kaspy and Glaser, 2020; Kathayat et al., 2021). Based on the above descriptions, we deduced that E. faecalis addition ameliorated gut microbial functions of APEC-challenged broilers partially through reppressing the virulence genes expression of cecal Escherichia, in addition to improving gut microbial composition/structure. In the future researches, the metagenomic analysis is anticipated to be employed to comprehensively evaluate the alterations in gut microbial functions of broilers fed with E. faecalis.
Conclusions
Supplementation of a native probiotic strain, E. faecalis TMBC 10513, attenuated APEC-induced poor growth performance and intestinal disruptions in broilers, which could be ascribed to its ability to directly inhibit APEC growth as well as improve intestinal immunity and microbial community in broilers. The above findings can expand our knowledge concerning the specific application of E. faecalis in controlling bacterial infections in chickens.
CRediT authorship contribution statement
Chong Ling: Writing – original draft, Conceptualization. Fei Yan: Methodology, Investigation. Kaixia Lin: Data curation. Hui Ye: Formal analysis. Qingyun Cao: Software. Zemin Dong: Resources. Changming Zhang: Validation. Jianjun Zuo: Supervision, Funding acquisition. Weiwei Wang: Writing – review & editing, Project administration, Funding acquisition.
Disclosures
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by the Guangdong Basic and Applied Basic Research Foundations (No. 2023A1515011112 and No. 2025A1515012884), National Natural Science Foundation of China (No. 32573263), Rural Science and Technology Correspondent Project of Guangzhou City (No. 2024E04J0277), and Key Research and Development Plan Project of Guangzhou City (No. 2025B03J0005).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2025.105725.
Contributor Information
Jianjun Zuo, Email: zuoj@scau.edu.cn.
Weiwei Wang, Email: wangweiwei@scau.edu.cn.
Appendix. Supplementary materials
References
- Agnieszka D., Jarzembowski T. From the friend to the foe-Enterococcus faecalis diverse impact on the human immune system. Int. J. Mol. Sci. 2024;25:2422. doi: 10.3390/ijms25042422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S., Jin T.E., Chang D.H., Rhee M.S., Kim H.J., Lee S.J., Park D.S., Kim B.C. Agathobaculum butyriciproducens gen. nov sp nov., a strict anaerobic, butyrate-producing gut bacterium isolated from human faeces and reclassification of eubacterium desmolans as Agathobaculum desmolans comb. Int. J. Syst. Evol. Microbiol. 2016;66:3656–3661. doi: 10.1099/ijsem.0.001195. nov. [DOI] [PubMed] [Google Scholar]
- Alkie T.N., Yitbarek A., Hodgins D.C., Kulkarni R.R., Taha-Abdelaziz K., Sharif S. Development of innate immunity in chicken embryos and newly hatched chicks: a disease control perspective. Avian Pathol. 2019;48:288–310. doi: 10.1080/03079457.2019.1607966. [DOI] [PubMed] [Google Scholar]
- An L., Li S., Chang Z., Lei M., He Z., Xu P., Zhang S., Jiang Z., Iqbal M.S., Sun X., Liu H., Duan X., Wu W. Gut microbiota modulation via fecal microbiota transplantation mitigates hyperoxaluria and calcium oxalate crystal depositions induced by high oxalate diet. Gut. Microbes. 2025;17 doi: 10.1080/19490976.2025.2457490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunins T.R., Erickson K.E., Chatterjee A. Transcriptome-based design of antisense inhibitors potentiates carbapenem efficacy in CRE Escherichia coli. Proc. Natl. Acad. Sci. USA. 2020;117:30699–30709. doi: 10.1073/pnas.1922187117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccouri O., Boukerb A.M., Farhat L.B., Zébré A., Zimmermann K., Domann E., Cambronel M., Barreau M., Maillot O., Rincé I., Muller C., Marzouki M.N., Feuilloley M., Abidi F., Connil N. Probiotic potential and safety evaluation of Enterococcus faecalis OB14 and OB15, isolated from traditional tunisian testouri Cheese and Rigouta, using physiological and genomic analysis. Front. Microbiol. 2019;10:881. doi: 10.3389/fmicb.2019.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford A., Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018;4:151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda C., Lopetuso L.R., Rizzatti G., Gibiino G., Cennamo V., Gasbarrini A. Actinobacteria: a relevant minority for the maintenance of gut homeostasis. Dig. Liver. Dis. 2018;50:421–428. doi: 10.1016/j.dld.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Boeder A.M., Spiller F., Carlstrom M., Izídio G.S. Enterococcus faecalis: implications for host health. World J. Microbiol. Biotechnol. 2024;40:190. doi: 10.1007/s11274-024-04007-w. [DOI] [PubMed] [Google Scholar]
- Cui Y.L., Zhang L.S., Wang X., Yi Y.L., Shan Y.Y., Liu B.F., Zhou Y., Lü X. Roles of intestinal parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022;369 doi: 10.1093/femsle/fnac072. [DOI] [PubMed] [Google Scholar]
- Cheng J.B., Hu J.L., Geng F., Nie S.P. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Well. 2021;11:1101–1110. [Google Scholar]
- Culp E.J., Goodman A.L. Cross-feeding in the gut microbiome: ecology and mechanisms. Cell Host. Microb. 2023;31:485–499. doi: 10.1016/j.chom.2023.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Carrasco J.M., Casanova N.A., Miyakawa M.E.F. Microbiota, gut health and chicken productivity: what is the connection? Microorganisms. 2019;7:374. doi: 10.3390/microorganisms7100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis R.S., Horn F. Enteropathogenic Escherichia coli, Samonella, Shigella and Yersinia: cellular aspects of host-bacteria interactions in enteric diseases. Gut. Pathog. 2010;2:8. doi: 10.1186/1757-4749-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.Y., Zhou W.Z., Li G.L., Liu X.S., Zhong P., Liu K.X., Liu Y., Wang D. Protective effects of sodium humate and its zinc and selenium chelate on the oxidative stress, inflammatory, and intestinal barrier damage of Salmonella Typhimurium-challenged broiler chickens. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.103541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R.B.R., Gill N., Willing B.P., Antunes L.C.M., Russell S.L., Croxen M.A., Finlay B.B. The intestinal microbiota plays a role in Salmonella-induced colitis independent of pathogen colonization. PLoS. One. 2011;6 doi: 10.1371/journal.pone.0020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Monzón E.G., Valencia-Mayoral P., Silva-Olivares A., Bañuelos C., Velázquez-Guadarrama N., Betanzos A. The Helicobacter pylori infection alters the intercellular junctions on the pancreas of gerbils (Meriones unguiculatus) World J. Microbiol. Biotechnol. 2024;40:273. doi: 10.1007/s11274-024-04081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Aizpurua O., de Rozas A.P., París N., Guivernau M., Jofre A., Tous N., Ng’ang’a Z.W., Alberdi A., Rodríguez-Gallego E., Kogut M.H., Tarradas J. Positive impact of early-probiotic administration on performance parameters, intestinal health and microbiota populations in broiler chickens. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.104401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspy I., Glaser G. Escherichia coli relA regulation via its C-terminal domain. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.572419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathayat D., Lokesh D., Ranjit S., Rajashekara G. Avian pathogenic Escherichia coli (APEC): an overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens. 2021;10:467. doi: 10.3390/pathogens10040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khongkool K., Taweechotipatr M., Payungporn S., Sawaswong V., Lertworapreecha M. Characterization and evaluation of Lactobacillus plantarum LC5.2 isolated from thai native pigs for its probiotic potential in gut microbiota modulation and immune enhancement. J. Microbiol. Biotechn. 2025;35:1–20. doi: 10.4014/jmb.2503.03028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladjouzi R., Taminiau B., Daube G., Lucau-Danila A., Drider D. The efficacy of the bacteriocinogenic Enterococcus faecalis 14 in the control of induced necrotic enteritis in broilers. Microbes. Infect. 2025;27 doi: 10.1016/j.micinf.2025.105477. [DOI] [PubMed] [Google Scholar]
- Li D., Zhang D.Y., Chen S.J., Lv Y.T., Huang S.M., Chen C., Zeng F., Chen R.X., Zhang X.D., Xiong J.X., Chen F.D., Jiang Y.H., Chen Z., Mo C.Y., Chen J.J., Zhu X.L., Zhang L.J., Bai F.H. Long-term alterations in gut microbiota following mild COVID-19 recovery: bacterial and fungal community shifts. Front. Cell Infect. Microbiol. 2025;15 doi: 10.3389/fcimb.2025.1565887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Gao Y., Zhao L., Chen Y., An S., Peng Z. Enterococcus faecalis lipoteichoic acid regulates macrophages autophagy via PI3K/Akt/mTOR pathway. Biochem. Biophys. Res. Commun. 2018;498:1028–1036. doi: 10.1016/j.bbrc.2018.03.109. [DOI] [PubMed] [Google Scholar]
- Lin X.Y., Xu M.C., Lan R.T., Hu D.L., Zhang S.P., Zhang S.W., Lu Y., Sun H., Yang J., Liu L.Y., Xu J.G. Gut commensal Alistipes shahii improves experimental colitis in mice with reduced intestinal epithelial damage and cytokine secretion. mSystems. 2025;10 doi: 10.1128/msystems.01607-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak Y., Byndloss M.X., Tsolis R.M., Bäumler A.J. Dysbiotic proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017;39:1–6. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Meng J., Wang W., Ding J., Gu B., Zhou F., Wu D., Fu X., Qiao M., Liu J. The synergy effect of matrine and berberine hydrochloride on treating colibacillosis caused by an avian highly pathogenic multidrug-resistant Escherichia coli. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.104151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi J.L., He T.N., Hu X.Y., Wang Z.H., Wang T.T., Qi X.L., Li K., Gao L., Liu C.J., Zhang Y.P., Wang S.Y., Qiu Y., Liu Z.Q., Song J., Wang X.M., Gao Y.L., Cui H.Y. Enterococcus faecium C171: modulating the immune response to acute lethal viral challenge. Int. J. Antimicrob. Ag. 2023;62 doi: 10.1016/j.ijantimicag.2023.106969. [DOI] [PubMed] [Google Scholar]
- Pan X., Kong R., Liu Q., Jia Z., Bai B., Chen H., Zhi W., Wang B., Ma C., Ma D. Probiotic Enterococcus faecalis surface-delivering key domain of EtMIC3 proteins: immunoprotective efficacies against Eimeria tenella infection in chickens. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.02455-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M., Cao Z.P., Shang P., Zhang H., Hussain R., Mehmood K., Chang Z.Y., Wu Q.X., Dong H.L. Comparative analysis of fecal microbiota composition diversity in Tibetan piglets suffering from diarrheagenic Escherichia coli (DEC) Microb. Pathog. 2021;158 doi: 10.1016/j.micpath.2021.105106. [DOI] [PubMed] [Google Scholar]
- Ren L.L., Cao Q.Y., Ye H., Dong Z.M., Zhang C.M., Feng D.Y., Zuo J.J., Wang W.W. Supplemental xylooligosaccharide attenuates growth retardation and intestinal damage in broiler chickens challenged by avian pathogenic Escherichia coli. Agric.-Basel. 2024;14:1684. [Google Scholar]
- Ruchaud-Sparagano M.H., Maresca M., Kenny B. Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell Microbiol. 2007;9:1909–1921. doi: 10.1111/j.1462-5822.2007.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schokker D., Hoekman A.J.W., Smits M.A., Rebel J.M.J. Gene expression patterns associated with chicken jejunal development. Dev. Comp. Immunol. 2009;33:1156–1164. doi: 10.1016/j.dci.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Shehata A.A., Tarabees R., Basiouni S., ElSayed M.S., Gaballah A., Krueger M. Effect of a potential probiotic candidate Enterococcus faecalis-1 on growth performance, intestinal microbiota, and immune response of commercial broiler chickens. Probiotics. Antimicrob. Proteins. 2020;12:451–460. doi: 10.1007/s12602-019-09557-2. [DOI] [PubMed] [Google Scholar]
- Song D., Wang Y.W., Hou Y.J., Dong Z.L., Wang W.W., Li A.K. The effects of dietary supplementation of microencapsulated Enterococcus faecalis and the extract of seed on growth performance, immune functions, and serum biochemical parameters in broiler chickens. J. Anim. Sci. 2016;94:3271–3277. doi: 10.2527/jas.2016-0286. [DOI] [PubMed] [Google Scholar]
- Song K., Li J., Tan Y.R., Yu J.Y., Li M., Shen S.Y., Peng L.Y., Yi P.F., Fu B.D. Xiaochaihu decoction treatment of chicken colibacillosis by improving pulmonary inflammation and systemic inflammation. Pathogens. 2023;12:30. doi: 10.3390/pathogens12010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swelum A.A., Elbestawy A.R., El-Saadony M.T., Hussein E.O.S., Alhotan R., Suliman G.M., Taha A.E., Ba-Awadh H., El-Tarabily K.A., Abd El-Hack M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: an updated overview. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L.Y.M., Chen Z.J., Shah N.P., El-Nezami H. Modulation of intestinal epithelial defense responses by probiotic bacteria. Crit. Rev. Food Sci. 2016;56:2628–2641. doi: 10.1080/10408398.2014.905450. [DOI] [PubMed] [Google Scholar]
- Wang K.L., Cao G.T., Zhang H.R., Li Q., Yang C.M. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019;10:7844–7854. doi: 10.1039/c9fo01650c. [DOI] [PubMed] [Google Scholar]
- Wang W.W., Ou J.S., Ye H., Cao Q.Y., Zhang C.M., Dong Z.M., Feng D.Y., Zuo J.J. Supplemental N-acyl homoserine lactonase alleviates intestinal disruption and improves gut microbiota in broilers challenged by Salmonella Typhimurium. J. Anim. Sci. Biotechnol. 2023;14:7. doi: 10.1186/s40104-022-00801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withanage G.S.K., Wigley P., Kaiser P., Mastroeni P., Brooks H., Powers C., Beal R., Barrow P., Maskell D. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect Immun. 2005;73:5173–5182. doi: 10.1128/IAI.73.8.5173-5182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.J., Wang W.Y., Kim I.H., Yang Y. Dietary hydrolyzed wheat gluten supplementation ameliorated intestinal barrier dysfunctions of broilers challenged with Escherichia coli O78. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Chen M., Han Z., Zhu C., Wu Z., Li J., Zhu G. Sfm fimbriae play an important role in the pathogenicity of Escherichia coli CE129. Microbiol. Res. 2025;16:160. [Google Scholar]
- Yin H.C., Zhang X.Y., Jiang X.J., Liu D. Characterization of the probiotic and functional properties of Enterococcus faecalis AQ10 isolated from chicken cecum. Lett. Appl. Microbiol. 2024;77 doi: 10.1093/lambio/ovae116. [DOI] [PubMed] [Google Scholar]
- Yin Y., Yang T.Z., Tian Z.Y., Shi C., Yan C.Q., Li H., Du Y., Li G.F. Progress in the investigation of the Firmicutes/Bacteroidetes ratio as a potential pathogenic factor in ulcerative colitis. J. Med. Microbiol. 2025;74 doi: 10.1099/jmm.0.001966. [DOI] [PubMed] [Google Scholar]
- Zhang G., Zahra A., Yang T., Guo Q., Sun Y., Zhang Y., Gao Y., Zhang Y., Wang M., Gong J., Huang H., Wang Z., Wang C., Jiang Y. Enterococcus faecalis strains derived from wild bird provide protection against Clostridium perfringens challenge in locally-sourced broilers. Front. Vet. Sci. 2025;12 doi: 10.3389/fvets.2025.1601605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.W., Mcwhorter A.R., Khan S., Willson N.L., Chousalkar K.K. Characterization of Lactobacillus spp. Isolated from layer hens as probiotic candidates. BMC. Vet. Res. 2025;21:416. doi: 10.1186/s12917-025-04847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang H.L., Yang D.H., Hao Q., Yang H.W., Meng D.L., Meindert de Vos W., Guan L.L., Liu S.B., Teame T., Gao C.C., Ran C., Yang Y.L., Yao Y.Y., Ding Q.W., Zhou Z.G. Lactobacillus rhamnosus GG triggers intestinal epithelium injury in zebrafish revealing host dependent beneficial effects. Imeta. 2024;3:e181. doi: 10.1002/imt2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Zhang Y., Wang Z., Dai J., Zhuge X. Exploiting membrane vesicles derived from avian pathogenic Escherichia coli as a cross-protective subunit vaccine candidate against avian colibacillosis. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.104148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.