Figure 1.
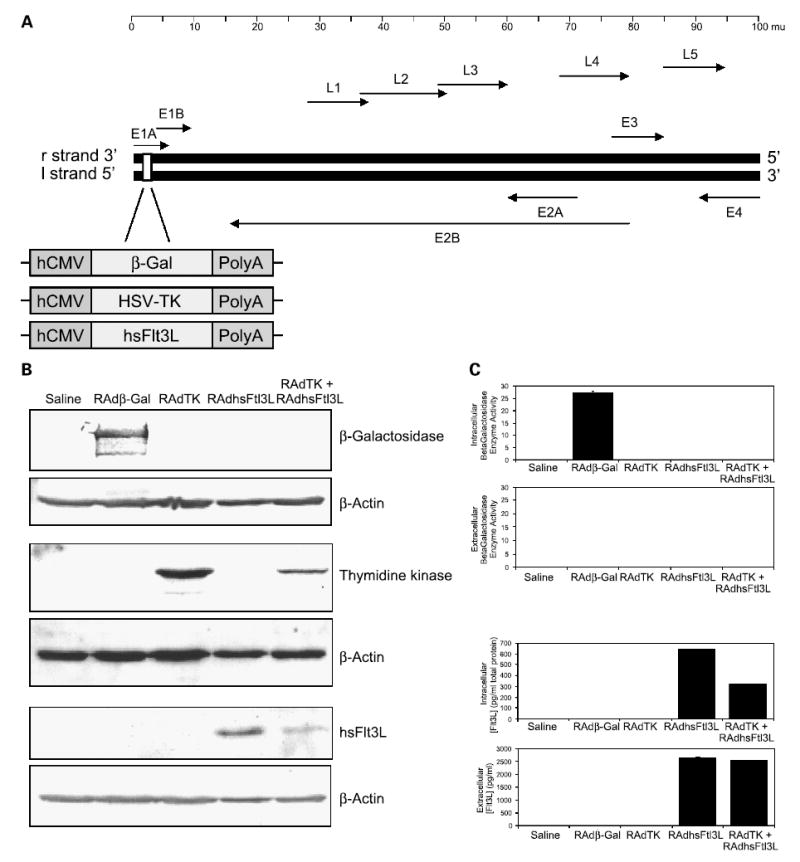
Generation and functional characterization of RAdFlt3L. A, a schematic diagram depicting the organization of the adenovirus type 5 genome (Ad5). Expression cassettes with Escherichia coliLacZ gene (β-Gal), HSV1-TK cDNA, or human soluble Flt3L cDNA (Flt3L) under the control of the hCMV promoter were created. These expression vectors were subsequently inserted into the E1 region of the adenovirus genome as shown, generating the recombinant adenoviral vectors RAdβgal, RAdTK, and RAdFlt3L. B, Western blot to detect RAd-generated protein from of CNS-1 cells supernatants and lysates that were mock infected, infected with RAdβgal, RAdTK, RAdFlt3L, or RAdTK + RAdFlt3L. C, β-galactosidase enzymatic activity assay from cell lysates and supernatants infected with viruses described in (B). Only intracellular protein extracts from RAdβgal-infected CNS-1 cells displayed significant β-galactosidase activity when compared with control samples (27.25 μg o-nitrophenol/μg protein/min). No secreted β-galactosidase activity was evident in any samples. D, ELISA to detect intracellular and secreted Flt3L expressed from cells infected with the viruses described in (B). Intracellular human Flt3L expression was only observed in RAdFlt3L (641.5 pg/mg total protein) and RAdTK + RAdFlt3L (320.8 pg/mg total protein)–infected CNS-1 cells. In addition, secreted human Flt3L was only detected in the media of RAdFlt3L and RAdTK + RAdFlt3L–infected CNS-1 cells at concentrations of 2,640.5 and 2,537.8 pg/mL, respectively.
