Figure 6. Effect of substrates on NEM inhibition of seHAS single Cys-to-Ala mutants.
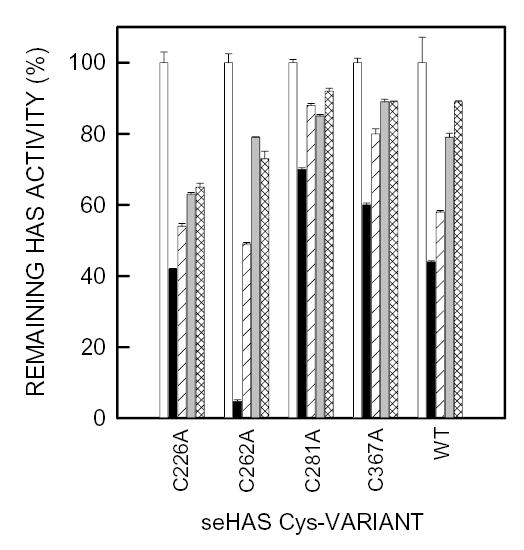
Different amounts of membrane protein, to compensate for their different levels of enzyme activity, containing wildtype or mutant seHAS were incubated at 4°C for 1 h in 50 mM phosphate buffer, pH 7.0 containing 10 mM MgCl2 and either PBS alone (open and solid bars) 1.5 mM UDP (diagonal line bars), 1.5 mM UDP-GlcNAc (gray bars), or 0.6 mM UDP-GlcUA (cross-hatched bars). The membranes were then incubated with 5 mM NEM (solid, diagonal line, gray and cross-hatched bars) or PBS alone (open bars) for 10 min at 4°C. DTE was then added to all samples to a final concentration of 25 mM, and the remaining HAS activity in each sample was determined as described in Methods. For each HAS variant, the activity without NEM (control) was set at 100% and the activity with NEM is expressed as a % of control. Results are the mean ± SEM for three separate experiments (n=3). The specific activities of the seHAS single Cys-mutants used (as a percent relative to the wildtype) were approximately 25% for C226A, 60% for C262A, 62% for C281A, and 140% for C367A (Kumari et al, 2002).
