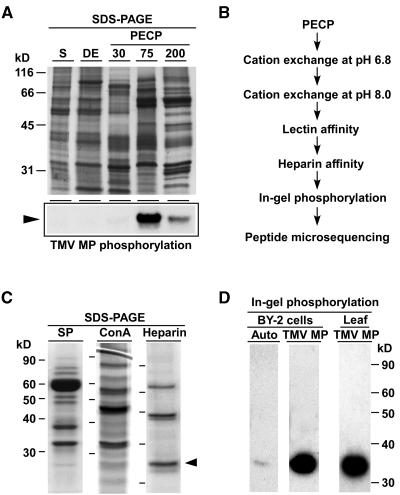
Figure 1.
Biochemical Isolation of a Tobacco PAPK.
(A) Enrichment of PAPK activity within the subfractions of a PECP preparation obtained using tobacco suspension cultured cells. Proteins from each fraction were resolved by electrophoresis in 10% SDS-PAGE gels and visualized by staining with GelCode Blue (top panel). The subcellular fractions include the supernatant (S), a 1% CHAPS extract of the cell wall pellets (DE), and PECP subfractions extracted with 30 mM (PECP30), 75 mM (PECP75), and 200 mM (PECP200) Ca2+ buffer. Each lane was loaded with 8 μg of protein. PAPK activity was determined by phosphorylation assays in which 1 μg of each fraction was added to assay mixtures containing TMV MP. Kinase assay reactions were resolved in 10% SDS-PAGE gels and autoradiography used to detected TMV MP phosphorylation (bottom panel, arrowhead).
(B) Biochemical protocol developed for PAPK purification. Phosphorylation assays were used to identify the chromatographic fractions containing peak enzyme activity for each step.
(C) Chromatographic purification of PAPK. Pooled PECP70 peak fractions were separated by cation exchange (SP; pH 6.8), lectin (Con A), and heparin columns. Proteins were resolved by electrophoresis in 10% SDS-PAGE gels and then stained with GelCode Blue. Protein loading volumes in each gel were 18, 25, and 35 μL for SP, Con A, and Heparin fractions, respectively. The arrowhead indicates ∼34-kD protein corresponding to the PAPK subsequently confirmed by in-gel phosphorylation assays.
(D) Purified heparin fraction, shown in (C), was used for in-gel autophosphorylation and TMV MP phosphorylation assays. For in-gel autophosphorylation, 20 μL of the heparin peak fraction was first resolved in 12% SDS-PAGE gels, then given denaturation/renaturation treatments, followed by incubation of the gel in complete assay buffer (2 mM DTT plus ATP mixture of 10 μCi/mL [γ32P]ATP) for 1 h. In-gel substrate phosphorylation assays were as above except that TMV MP was included at ∼300 μg/mL gel. PAPK was also purified from tobacco leaves using the same purification protocol as (B). Peak fractions were combined, concentrated to ∼80 μL, and 5-μL aliquots used for each in-gel TMV MP phosphorylation assay.