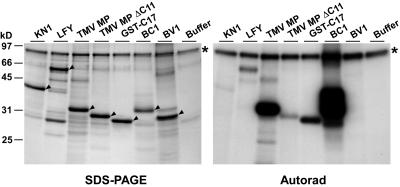
Figure 10.
PAPK1 Phosphorylates TMV MP in a Similar Manner to Tobacco PAPK.
Recombinant Arabidopsis PAPK1 phosphorylates full-length TMV MP and BDMV BC1 strongly and LFY less extensively. The C-terminal deletion mutant (TMV MPΔC11) and the C-terminal peptide (GST-C17) derivative of TMV MP showed reduced phosphorylation by Arabidopsis PAPK1 in a manner similar to that observed for tobacco PAPK. Among the tested proteins, KN1 and BDMV BV1 were not phosphorylated by Arabidopsis PAPK1. Negative control: phosphorylation reaction in the absence of substrate (buffer) shows GST-PAPK1 (indicated by asterisk) as an autophosphorylating band. Predicted molecular mass of GST-PAPK1 is ∼82 kD. Target substrate protein in each lane is marked by arrowheads. Phosphorylation assays were performed essentially as described for Figures 3 and 4, except that 0.4 μg of purified GST-PAPK1 was used in 50 μL of kinase reaction mixture. The assay was terminated by adding 10 μL of 6× SDS sample buffer, and 40 μL of each reaction was resolved on 12% SDS-PAGE. The gel was stained with GelCode Blue (SDS-PAGE), dried, and exposed to x-ray film (Autorad).