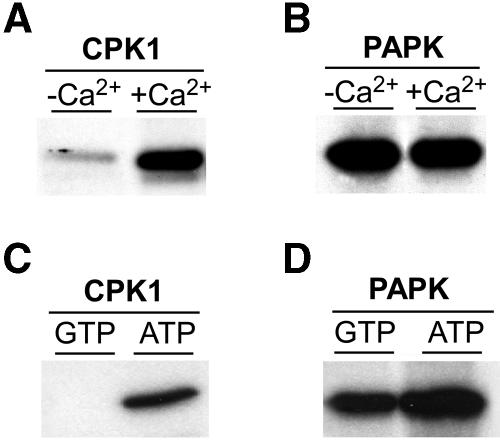
Figure 2.
PAPK Exhibits Calcium-Independent Activity and Uses ATP or GTP as a Phosphate Donor.
(A) Control experiment employing pumpkin CPK1 demonstrated calcium-dependent phosphorylation of TMV MP. Kinase activity was assayed using 50 μL reaction buffer, plus 20 ng of purified recombinant enzyme and 1 μg of TMV MP. Reaction buffer contained 50 mM HEPES, pH 7.5, 10 mM MgCl2, 2 mM DTT, 2 mM EGTA, and 60 μM ATP mixed with 4 μCi of [γ32-P]ATP (3000 Ci/mmol), supplemented with 2.2 mM CaCl2.
(B) PAPK phosphorylated TMV MP in a calcium-independent manner. Kinase assays were performed in 50 μL of reaction buffer, plus 5 μL of heparin peak fraction and 1 μg of TMV MP ± 2.2 mM CaCl2.
(C) Pumpkin CPK1 uses ATP but not GTP as a phosphate donor. Reaction buffer was as above, except that it contained 4 μCi of [γ32P]GTP (3000 Ci/mmol) mixed with 60 μM ATP.
(D) PAPK can use GTP or ATP as the phosphate donor. Reaction buffer was as above, except that it contained 4 μCi of [γ32P]GTP (3000 Ci/mmol) mixed with 60 μM ATP.